
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
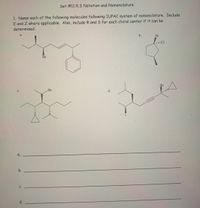
Transcribed Image Text:Set #11 R,S Notation and Nomenclature
1. Name each of the following molecules following IUPAC system of nomenclature. Include
E and Z where applicable. Also, include R and S for each chiral center if it can be
determined.
a.
b.
Br
Br
Br
Br
d.
a.
b.
С.
d.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Shown below is Streptomycin, and Neomycin B. Circle and label as many functional groups in these molecules as you can. a. Label each chiral carbon in Streptomycin. How many total stereoisomers exist for Streptomycin? b. Label each chiral carbon in Neomycin B. How many total stereoisomers exist for Neomycin B?arrow_forwardUse flat representation of rings, not chair in the drawing. Determine the most and least stable. Consider the most stable chair for each of these isomers, and then draw the most stable and least stable isomer based on a comparison of the best chair for each one.arrow_forwardPlease help me solve the first set of questionsarrow_forward
- How many stereoisomers estuary for this molecule? Show how you know. A. 8 B. 3 C. 4 D. 6arrow_forward1. How many chiral (stereocenters) centers are in the following molecules? Recall that a chiral center will have a carbon atom bonded to four different substituents. Please circle the chiral centers. Structure I HO... III. Br !!! СОН Structure II NH2arrow_forward5) relationship between the pairs of structures. NOTE: Each term may be used more than Choose the term from the five terms listed below that BEST describes the once and not all terms need be used. Identical Diastereomers Enantiomers Constitutional isomers Not isomers CH3 CH3 ÇI H3C-Br CH3 Br -CI H,C. D-H H3C- Br H- -D Br CH3 -CI ČH3 OH H3C, CHO OHC, OH OH H. HO CHO OHC CH3 H. OH ÓHarrow_forward
- 10. Provide a structure of an isomer that has the molecular formula C5H8O2 and fits the criteria described below. Note that all of the structures must be neutral and not have any formal charges on any of the atoms. a) Contains an ester and an alkene b) A chiral compound that contains a ring, ether and ketone. c) Contains an alcohol and an alkyne. d) A meso compound that contains two alcohols.arrow_forwarda. b. For the following two pairs of molecules, (1) Draw out the chair conformation for each molecule, flip the ring if it is possible. (2) Compare both molecules to circle out which one is more stable. (3) Identify their relationship as: constitutional isomer, conformational isomer, stereoisomer or identical. (4) Find all the chiral center on each molecule and label them. Br.. Ax < and and H. H H Br H Harrow_forwardQ3: Describes the relationship (identical, constitutional isomers, enantiomers or diastereomers) of each pair of compounds below. ག H CH3 OH OH CH3 H3C OH OH OH ////////// C CH3 CH3 CH3 CH3 H3C CH 3 C/III..... Physics & Astronomy www.physics.northweste COOH H нош..... H 2 OH HO CH3 HOOC H CH3 CH3 CH3 Br. H H Br and H H H Harrow_forward
- Chemistryarrow_forwardОН ÇH3 ÇH3 ÇH3 H;C-O CH3 10 H3C-0 CH3 ОН This compound is called type your answer..arrow_forwardParaphrasing .ewriting Tool Car note OWL Table Pirate Ship BLACKBOARD [Review Topics] (References) Indicate whether the pair of structures shown represent stereoisomers, constitutional isomers, different conformations of the same compound, or the same conformation of a compound viewed from a different perspective. Note that cis, trans isomers are an example of stereoisomers. но. HO ČI Br Br CI Br Br Submit Answer Retry Entire Group 8 more group attempts remaining (Previous Next Save and Exit étv O 14 MacBook Air DD F9 FB 30 888 F7 F6 F4 * & 2$ 8 9. 7 4 5 Y R L H J V .. .- V Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning