
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
(d) Illustrate and briefly explain the mechanism of reaction II.
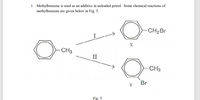
Transcribed Image Text:1. Methylbenzene is used as an additive in unleaded petrol. Some chemical reactions of
methylbenzene are given below in Fig. 5.
CH2BR
X
CH3
II
CH3
Br
Y
Fig. 5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How would you account for the following :(a) Electrophilic susbstitution in case of aromatic amines takes place more readily than benzene.(b) Ethanamide is a weaker base than ethanamine.arrow_forwardProvide the mechanism of the redica reaction below (b)arrow_forward(b) Alkene 6 undergoes the reaction sequence shown below to generate 7, answer ALL parts (i) to (iii) 1) Hg(OAc)2, H₂O 2) NaBH4 7 (C11H14O) 6 (i) Identify compound 7 and give a mechanism for the reaction with Hg(OAc)2 and water. [A mechanism for the reaction with NaBH4 is not required] (ii) With reference to your mechanism, explain the regioselectivity of the reaction. (iii) What would the product be if the second step was performed using deuterated sodium borohydride (NaBD4).arrow_forward
- 5.40. Give plausible products (or just one product) for each of the following reactions, or tell if no reaction is expected. (a) PDSO, + MgS > (b) (CH,),Hg + CaF, → 1. 1.arrow_forward3. How could you make the following compounds from the indicated starting materials and anything else you need? (a) (b) (c) OH from from from Br Brarrow_forwardNeed answer step by steparrow_forward
- For EACH of the following reactions (a)-(d) provide a curved arrow mechanism and where appropriate briefly comment on any issues of selectivity that arise. (a) (b) (c) CHO MeO OMe 3 OH MeO NH OEt Me (i) Ph3P=CBr2 (ii) "BuLi (2 equivs) (iii) Mel + H₂SO4 (cat) OMe H₂O Me3Si * TfO OMe O MeCN CO₂Me MeO CsF, NaHCO3 Me COMe OMearrow_forward(3) Mechanism. Provide the mechanism for the following reaction. OH H₂SO4 (cat.) OHarrow_forward3.) Outline an electron-pushing mechanism for the following reaction.. + ㅅ NaOH, H₂Oarrow_forward
- 4. (a) (i) Draw the mechanism with three-dimensional conformations and identify the product for the solvolysis (SN1) reaction between compound (A) and acetic acid (CH3CO₂H). Me (A) OTS H (B) OTS (ii) Predict, with reasoning, whether compound (A) or compound (B) will undergo solvolysis faster.arrow_forward(b) Strongly electron-withdrawing substituents on benzene rings are meta-directing. Show by drawing the resonance structures for the various possible intermediates why this is the case using nitrobenzene as an example.arrow_forward3. Benzalacetophenone can be nitrated to give the mononitro derivative.(a) Provide the structure of the mononitro derivative.(b) If a second nitro group is introduced, where would it enter? Give the structure of theproduct.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY