
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
1. L-aspartic acid + phosgene (COCl2)= ?
2.Draw the (S)-enantiomer of two sweeteners intermediate 4 + intermediate 5 = two sweeteners intermediate 6. (Intermediate 4 is the image provided)+ (Intermediate 5 is the product of number 1)= Intermediate 6
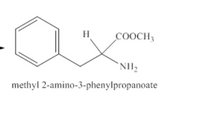
Transcribed Image Text:H
COOCH3
`NH2
methyl 2-amino-3-phenylpropanoate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw all of the possible stereoisomers of the compound shown. Group them as enantiomeric pairs, or indicate any meso compounds. Label all stereocenters with the absolute stereochemistry (R or S).arrow_forwardzarrow_forwardWhat type of product is this? A racemic mix? Achiral or meso? diastereomers?arrow_forward
- a. D-(+)-mannose is a diastereomer of glucose that is important in protein glycosylation. Find the Fischer projection of D-(+)-mannose in your textbook (Chapter 23), and draw it very clearly below. b. Draw a box around the chiral center furthest from the aldehyde/ketone. (This is the carbon which can be used to determine whether the carbohydrate is D- or L-.) c. Clearly label each of the chiral centers on D-(+)-mannose with its correct (R) or (S) configuration. d. D-(+)-mannose has two different Haworth projections-one that is a and one that is ß. Draw both Haworth projections for D-(+)-mannose, and label each as either a or B. e. In addition to Benedict's test, several other chemical tests can provide information about the type(s) of carbohydrate present in a given solution. These include Barfoed's test, Seliwanoff's test, and Bial's test. Use the internet to research what each test's possible results are, and what those results tell you about the carbohydrate's structure. Note: You…arrow_forward23) Label the following pair as a/an enantiomer, diasteromer, structural isomer or identical C₂H5 НО (I) C₂H5 Н. (II) (III) HO НО OH -C2H5 он || -C2H5 OH НО Н. но. Н OH C₂H5 OH C₂H5 H H OH ОНarrow_forwardPart II: Short answer ) 1. Draw (a) an enantiomer, and (b) a diastereomer of the following compound OH HO -CH₂ CH₂CH₂arrow_forward
- 1. Carbohydrates classification. 2. Write down the reactions: a) α,D-Glucopyranose + C2H5OH → b) D-Glucose + [Ag(NH3)2]+ → c) D-Glucopyranose + (CH3CO)2 O → d) D-Glucopyranose + CH3I → e) D-Glucose + HNO3 → f) D-Glucose + H2 → g) Lactose formation h) Sucrose hydrolysis 3. Write down the formula of β,D-galactopyranosearrow_forward7arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY