
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
1. How did the temperature change throughout the experiment? Use relative terms like increase, decrease or no change, to explain.
2. What phase change is occurring during the addition of 20 to 100 calories?
3. What is happening to the water molecules in the solid-state during the addition of the first 20 calories, and in the liquid state during the
addition of 100 to 200 calories?
4. What phase change is occurring during the addition of 200 to 700 calories?
5. Write a paragraph that explains what latent heat is and use latent heat to explain what the ice, liquid, and gas experienced throughout the experience
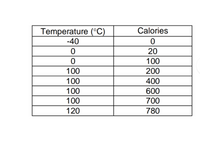
Transcribed Image Text:Temperature (°C)
Calories
-40
20
100
100
200
100
400
100
600
100
700
120
780
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- make 100.0 mL of either 1.0 M or 0.10 M ethanol (aqueous). Start with a stock ethanol solution that is 95.0% ethanol by mass and has a density of 0.789 g/mL.arrow_forwardWhy different amounts of water and chlorophyll solution are being mixed in the six different beakers. What does it mean when there is more water compared to more solutions?arrow_forwardHow do you find the percent of water hydration?arrow_forward
- what happens when you put a mixture of rubbing alcohol and water on baking soda?arrow_forwardI need help answering these 2 questions.Thank YOUarrow_forwardWhat chemical concentration is being analyzed in this experiment. a. The amount of sodium bicarbonate in baking soda. b. The amount of acetic acid in commerical vinegar. c. The amount of citric acid in commerical lemon juice. d. The amount of lead in water.arrow_forward
- In chem lab when creating aspirin, how does a melting point range for pure aspirin compound differ from impure? Explain the meaning of value and range of melting point. How does the meaning of value melting point and range of melting pointarrow_forwardwhat would be happen if you add 5g of sugar instead of 0.5g of sugar to 5 ml of waterarrow_forward1. How much glucose do you need to make 500 ml of 5% glucose? 2. What is the molarity of the glucose solution made in #1?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY