
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Calculate the values of all external reactions.
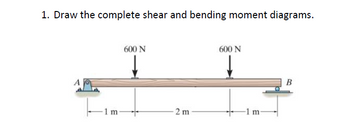
Transcribed Image Text:1. Draw the complete shear and bending moment diagrams.
A
1 m
600 N
2 m
600 N
-1 m-
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- a- Schematize the equilibrium diagram for the A-B system, based on the following data:Melting point of A= 700ºC; Melting point of B = 1,000ºC; eutectic temperature = 500ºC; Composition of the liquid in equilibrium at eutectic temperature = 30%A and 70%B; Solubility at 500ºC, B at A = 15% and A at B = 20%. b- What is the type of steel and the carbon content according to the ABNT classification for the following steels: ABNT 1040, ABNT 1130, ABNT 5160 e ABNT 8620. c- Why is the Fe-Fe3C phase diagram considered metastable?arrow_forwardDefine the term stable equilibrium?arrow_forwardGive an example of phase equilibrium and chemical equilibrium?arrow_forward
- te wit une de Answer true or false: The orientation of the neutral axis of a beam undergoing pure bending is dependent only on the geometry of the beam.arrow_forwardAn alloy consisting of completely soluble cadmium (Cd) and zinc (Zn) in the liquid state, but neither of them dissolves in each other in the solid state. the table shown below shows the solidification temperatures for various alloys of cadmium and zinc. 1. Draw the equilibrium diagram according to the information given and data in the table and indicating all important temperature and phases. 2. Find the percentage of each phases and percentage of constituents of the alloy that contain 60 % Zn and at a temperature 300 °C. 3. Find the melting point for the following alloys 20 % Cd, 80% Cd 4. Draw the internal structure, noting the phases of the following alloys A) 30 % Cd at 290 °C b) 60 % Cd at room temperature. % of Zinc in alloy Start of solidification ("C) End of solidification ("C) 0 10 14 20 30 40 50 60 321 290 266 275 293 310 328 345 70 80 90 100 362 390 401 419 266 266 266 266 266 266 266 266 266 266 266 266arrow_forward6.8 Suppose that a cast Al-1 wt% Si alloy, contains equilibrium Si particles 1 µm in diameter. This alloy is to be heat treated by first putting all of the Si particles back into solution. (a) Draw a set of schematic curves indicating what will happen during this process. Temperature °C (b) Indicate the initial conditions, the final conditions (once a new equilibrium is reached) and the boundary conditions during dis- solution. (c) Estimate how long the alloy should be held at 550°C so as to ensure that all of the Si is dissolved. Al-Si 1500 1300 1100 900 700 500 300 660.452°C 0 -(A1) 10 AI 12.6 10 20 20 30 30 Atomic Percent Silicon 40 50 Thom L 60 Them 577+1°C 40 50 60 Weight Percent Silicon 70 70 80 80 90 90 100 1414°C (Si)- 100 Siarrow_forward
- 3. Explain different types of equilibriums with examples.arrow_forwardPhases that exist on the right side of eutectic point of an invariant reaction line are called: Select one: a. post-phase b. hypo-phase c. pro-phase O d. hyper-phasearrow_forwardHow can the shear force, be determined from the method of sections andthe equations of equilibrium?arrow_forward
- What is neutral equilibrium?arrow_forwardParvinbhaiarrow_forwardConsider the phase diagram below. The three points A, B, and C are at concentrations of 27, 31.9, and 33.8 wt% Ni respectively. The ends of the tie line are at C1 = 25% wt% Ni and C2 = 35 wt% Ni. What are the weight fractions of the alpha phase at A and the L phase at B, as well as the alpha phase/L phase ratio at C? T(°C) 1300-L (liquid) 1200 20 ABC L + a C1 30 S C2 liquidus 40 L + a solidus α (solid). O a. Walpha=0.12; WL=0.41; Walpha/WL = 9.41 O b. Walpha=0.27; WL-0.26; Walpha/WL = 11.11 O c. Walpha=0.15; WL-0.26; Walpha/WL = 11.80 Od. Walpha=0.20; WL-0.31; Walpha/WL = 7.33 50 wt% Niarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY