
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
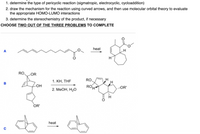
Transcribed Image Text:1. determine the type of pericyclic reaction (sigmatropic, electrocyclic, cycloaddition)
2. draw the mechanism for the reaction using curved arrows, and then use molecular orbital theory to evaluate
the appropriate HOMO-LUMO interactions
3. determine the stereochemistry of the product, if necessary
CHOOSE TWO OUT OF THE THREE PROBLEMS TO COMPLETE
heat
A
RO,
LOR
КН, THF
RO,
в
-OH
ROH
...OR'
2. МеОн, Н,о
OR'
heat
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ] Draw the structure of Diels-Alder cycloadduct formed via an endo 3. [a, transition state structure involving the diene and dienophile shown here. [b Formulate the endo transition structure that leads to the product. [c is observed. Does it correspond to a primary or a secondary KIE? Explain. [d. the 'inverse' variety? Explain. -CO2ET ]A KIE ] Is the KIE of the 'normal' orarrow_forwardbest condition for the reaction (1r,2s)-1 bromo-2methylcyclohexane->(s)3-methylcyclohex-1enearrow_forward[References] The two alkenes below react with HI at different rates. CH3 CH3CH=CHCH3 and CH;ċ=CHCH3 Draw the structural formula of the MAJOR product formed by the alkene having the HIGHER reaction rate. You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. In cases where there is more than one answer, just draw one. C P opy aste Previous Next ChemDoodle®arrow_forward
- Draw the structure of (Z)-1-bromo-1-chloro-1-butene. • Consider E/Z stereochemistry of alkenes. • You do not have to explicitly draw H atoms. AAVII ? ChemDoodle [F Ⓡarrow_forward44a) Draw the bond-line structure of 1,4-dimethyl-cyclohexa-1,3-diene here: STRUCTURE: b) Circle all the sp²-hybridized carbons in your drawing of the structure. c) In the spaces below, draw two potential products from a chlorination reaction (addition of Cl2) with your first structure Product 1: Product 2:arrow_forward[References) Draw the line-angle formula for the most stable carbocation with a molecular formula of: C3H,+ • You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • If there are alternative structures, draw the most stable one. P opy aste ChemDoodlearrow_forward
- Markovnikov Hydrohalogenation – HX (SYN & ANTI) :CI: H-CI: Scenario A :Cl: H. A В H-ci: H-CI: ..O :Cl: Scenario B CI: D 1. Fill in the curved arrows to show the mechanism of the reaction in Scenario A & B. 2. What is the hybridization and shape of the intermediate carbocation? How will the hybridization and shape of the intermediate carbocation affect the structure of the final product?arrow_forward2. Our knowledge of chair conformations allows us to use them to determine a great deal about a reaction's mechanism. In particular, using a t-butyl group to 'fix' a cyclohexane in a single conformation can help us learn about the spatial requirements and energetics of a reaction. Consider the following two reaction products: a. NO NID ALOH 1) NaCN 2) H+ 1) NaBH4 2) H3O+ OH Group -H -CN -OH A Value (kcal/mol) Defined as 0 0.2 kcal/mol 1.0 kcal/mol Draw the most stable chair conformation for each of the two products. b. Based on the A values in the table above, do the two products have the most stable conformation at the new stereocenter?arrow_forwardWhat is the expected major product for the following reaction? O IV I OCH 3 |||||... + enantiomer || 1. Hg(OAc)2, CH3OH 2. NaBH4 НО. OH ... H3CO. + enantiomer ||| IV H3CO Varrow_forward
- 5. Consider the reaction below: ÇI CH3 CH CHCH3 CH, CHCHCH, + ci CH3 (a) State the type of bond cleavage involved. (b) Compare the stability of the product to (CH3)2CHCH;CH2 and explain.arrow_forward4. Draw the mechanism that accounts for the formation of the product under the conditions shown. You may not add any other reagents. Be sure to show: all intermediate structures that occur in the course of the mechanism, any important resonance structures that play a role in the process, what if anything is added or lost in each step, and all formal charges on the structures. CHO H+ HO-C-H HO OH H-C-OH CH,OH НО 1 2 = 3arrow_forwardHow many atoms of each hybridization state are there (sp,sp2, sp3)?What would be the result after the shown molecule is carried through the 4 step sequence? Show each intermediate and stereochemistry 1. 1eq. MeLi, then H2O workup 2.TsCl/pyr 3.NaOtBu/BuOH 4.OsO4/NaHSO3 And What is the relationship between the isomeric end products from them?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning