
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

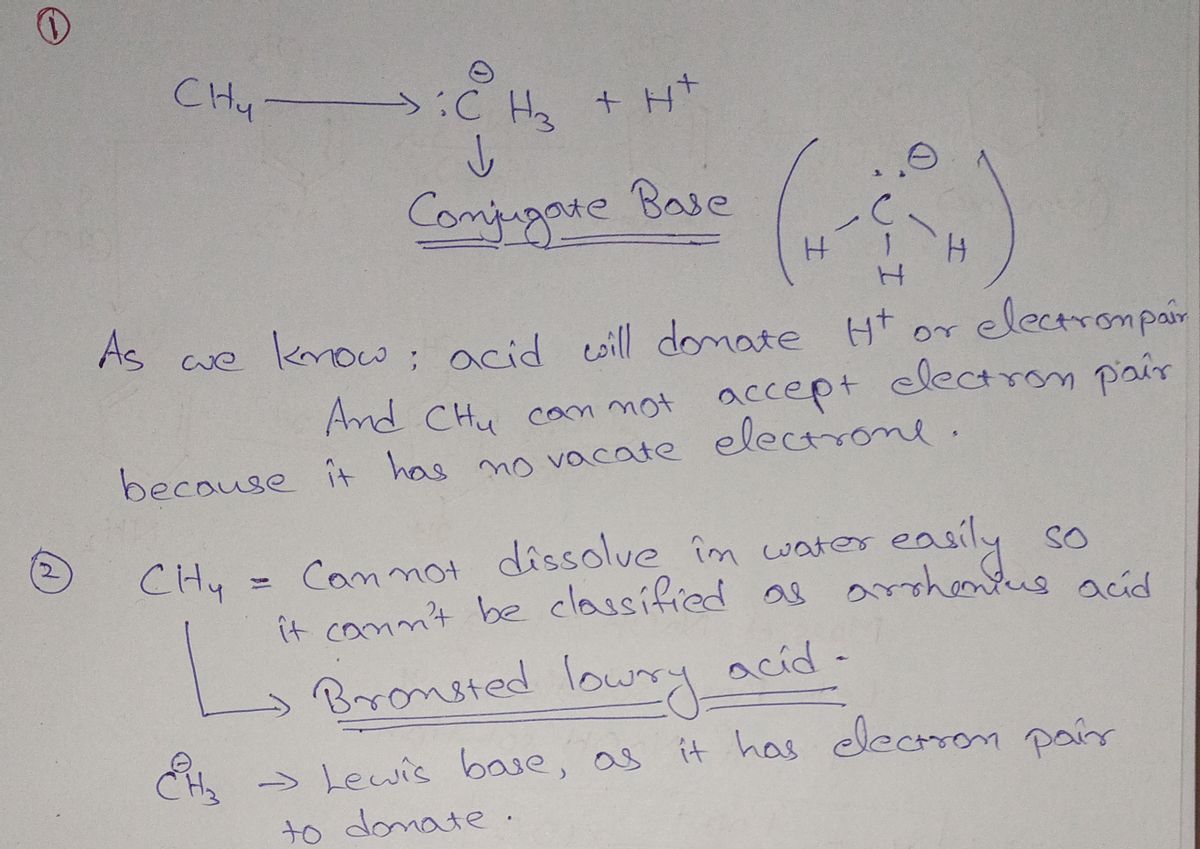
Transcribed Image Text:1. Consider Lewis structure for methane (CH4).
a. If methane were to behave as an acid (if possible), draw the Lewis structure of its
resulting conjugate base.
b. Reflecting on your answer for Part A and using the three acid-base models above,
would you predict the conjugate base would form? (Does this situtation make
sense)?
c. If methane were to behave as a base (if possible), draw the Lewis structure of its
resulting conjugate acid.
d. Reflecting on your answer for Part A and using the three acid-base models above,
would you predict the conjugate acid would form? (Does this situtation make
sense)?
Expert Solution
arrow_forward
Step 1
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6. In Group C, do all four compounds appear to be molecular, ionic, or molecular acids? Based on this answer, would you expect them to dissociate? 7. How do you explain the relatively high conductivity of tap water compared to a low or zero conductivity for distilled water? 8. Did aqueous methanol, CH,OH, have the same conductivity value as aqueous ethylene glycol, CH.O:? Explain.arrow_forwardOrder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. species ОН IO C10₂ HCOOH HCIO₂ HIO HCOO relative pH of 0.1 M aqueous solution (Choose one) 7 (Choose one) 2 1 (lowest) (Choose one) (Choose one) X Ś olo 18 Ararrow_forwardA weak base has a Kb = 1.5 x 109. What is the value of the pKa for the conjugate acid? Enter your answer, rounded to the correct number of significant figures, into the box below. Do not use scientific notation. If your answer is a decimal, please include a 0 before the decimal point. pka = type your answer...arrow_forward
- a. The formula for the conjugate base of HSO3 is T b. The formula for the conjugate acid of H₂PO4 isarrow_forwardAmmonia is a weak base and acetic acid is a weak acid. Which statement is true of a solution of ammonium acetate? a. It is weakly acidic. b. It is weakly basic. c. It is strongly acidic. d. It is neutral. e. We cannot predict its acid-base properties without more information.arrow_forwardOrder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. species ΙΟ HF HIO F ОН NO₂ HNO₂ H₂O relative pH of 0.1 M aqueous solution (Choose one) (Choose one) 3 5 8 (highest) (Choose one) 2 (Choose one) X Śarrow_forward
- Order these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. species relative pH of 0.1 M aqueous solution + C6H5NH3 HCOOH 3 2 HCOO (Choose one) H₂O HONH3 5 + (Choose one) C6H5NH2 (Choose one) H₂O+ (Choose one) HONH2 8 (highest)arrow_forwardOrder these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. relative pH of 0.1 M aqueous solution species ? 103 |(Choose one) ▼ OH |(Choose one) ▼ H,0 4 HC,04 6. HIO, 1 (lowest) NO2 |(Choose one)arrow_forward3arrow_forward
- Order these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. relative pH of species 0.1 M aqueous solution HC₂04 7 + H₂O* 1 (lowest) H₂PO4 (Choose one) H₂PO4 4 103 (Choose one) HIO₂ H₂C₂O4 (Choose one) H₂O (Choose one)arrow_forwardGiven: HCHO_2(aq) + H_2O = CHO_2- (aq) + H_3O+ a. What is the conjugate of HCHO_2(aq)? b. Between these two species HCHO_2(aq) and its conjugate, what is the conjugate acid? c. What is the conjugate base?arrow_forwardWhat is the best rationale for the difference in acidity in a 2,2-difluoro isomer and a 3,3-difluoro isomer. A. The more acidic compound has a hydrogen attached to a more electronegative atom B.The more acidic compound has a hydrogen attached to a larger atomic radius atom C. The more acidic compound has a more stable conjugate base because of addition resonance structures stabilizing the charge D. The more acidic compound has a stronger molecular dipole that increases the polarization of the acidic hydrogen E. The more acidic compound has a greater amount of s-character in the acidic bond.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY