
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
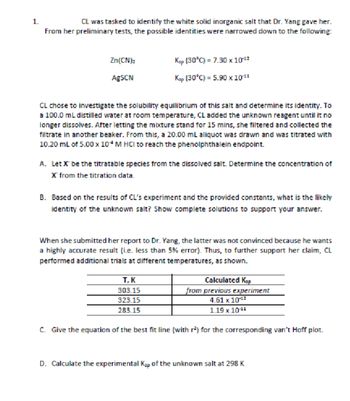
Transcribed Image Text:1.
CL was tasked to identify the white solid inorganic salt that Dr. Yang gave her.
From her preliminary tests, the possible identities were narrowed down to the following:
Zn(CN)₂
Ksp (30°C) = 7.30 x 1012
AgSCN
Ksp (30°C) = 5.90 × 10 11
CL chose to investigate the solubility equilibrium of this salt and determine its identity. To
a 100.0 mL distilled water at room temperature, CL added the unknown reagent until it no
longer dissolves. After letting the mixture stand for 15 mins, she filtered and collected the
filtrate in another beaker. From this, a 20.00 mL aliquot was drawn and was titrated with
10.20 mL of 5.00 x 10 M HCI to reach the phenolphthalein endpoint.
A. Let X be the titratable species from the dissolved salt. Determine the concentration of
X from the titration data.
B. Based on the results of CL's experiment and the provided constants, what is the likely
identity of the unknown salt? Show complete solutions to support your answer.
When she submitted her report to Dr. Yang, the latter was not convinced because he wants
a highly accurate result (i.e. less than 5% error). Thus, to further support her claim, CL
performed additional trials at different temperatures, as shown.
T, K
Calculated Ksp
303.15
323.15
from previous experiment
4.61 x 10-¹2
283.15
1.19 x 10-¹1
C. Give the equation of the best fit line (with r²) for the corresponding van't Hoff plot.
D. Calculate the experimental Kap of the unknown salt at 298 K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If a 0.100 M solution of NaOH is added to a solution containing 0.200 M Ni2+, 0.200 M Ce3+, and 0.200 M Cu2+, which metal hydroxide will precipitate first? Ni(OH)2, Ksp = 6.0 x 10–16 Cu(OH)2, Ksp = 4.8 x 10–20 Ce(OH)3, Ksp = 6.0 x 10–22 A) All metal hydroxides precipitate at the same time. B) Ce(OH)3 C) Ni(OH)2 D) Cu(OH)2arrow_forwardThe solubility product constant for Ba(IO3)2 at 30.0°C is 7.8 x 10^-9. Determine the molar solubility if 65.0 g of Ba(NO3)2 is dissolved in 2.5 L of a Ba(IO3)2 saturated solution.arrow_forwardG.137.arrow_forward
- A technician mixes 0.10 L of 0.10 M NaCl with 0.30 L of 0.20 M AgNO3. Which of the following statements is correct? Ksp = 1.6 × 10-10 for AgCl. Question 47 options: 1) The solution is at equilibrium. 2) The solution is not at equilibrium and no precipitate will form. 3) AgCl will dissolve until the solution is saturated. 4) AgCl will precipitate until the solution is saturated. 5) None of above.arrow_forwardA solution contains 8.49×10M sodium carbonate and 9.73×10 M potassium hydroxide. Solid calcium nitrate is added slowly to this mixture. What is the concentration of carbonate ion when hydroxide ion begins to precipitate? [carbonate] = Marrow_forwardA solution contains 1.03×10 M sodium cyanide and 8.68×10M potassium sulfate. Solid silver nitrate is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of silver ion when this precipitation first begins? [Ag*] =[ Marrow_forward
- A solution contains 5.35x10-³ M sodium hydroxide and 1.31x10-² M potassium sulfide. Solid iron (III) acetate is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula B. What is the concentration of iron(III) ion when this precipitation first begins? [Fe³+] = Marrow_forwardSolid sodium hydroxide is slowly added to 125 mL of a 0.0449 M chromium(III) acetate solution. The concentration of hydroxide ion required to just initiate precipitation is M.arrow_forwardA solution contains 1.13×10-² M nickel(II) acetate and 7.82x10-3 M zinc nitrate. Solid potassium cyanide is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of cyanide ion when this precipitation first begins? [CN] = Marrow_forward
- 6arrow_forwardPlease don't provide handwriting solutions...arrow_forwardA solution contains 1.23x10-2 M ammonium sulfite and 8.98x10-3M potassium carbonate. Solid barium acetate is added slowly to this mixture. A. What is the formula of the substance that precipitates first? formula = B. What is the concentration of barium ion when this precipitation first begins? [Ba2") = Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY