
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
1. calculate the delta Hf in Kj of NH4Cl (s) ussing hess law. write out all reactions.
calculate the % error, accepted value is -314.4 kj/,ol
is it exothermic or endothermic? explain.
Transcribed Image Text:D2L S-23 Enthalpy 2 Lab Packe X D2L S-23 Enthalpy 2 Lab Proce X
个
Apps
35°F
Download
✰ uscga.desire2learn.com/d2l/le/content/29072/viewContent/487199/View
United States Coast... D2L Homepage - Unite... Mail - Bangsoy, Arl... CGAportal
in the laboratory. ThS OVET All TEACTION IS Tepresented by.
rxn 1:
rxn 2:
rxn 3:
rxn 4:
overall rxn:
Digital Resources for Cher X
Print
You have viewed this topic
Last Visited Feb 6, 2023 10:31 PM
Chemistry: An Atoms-Foc X
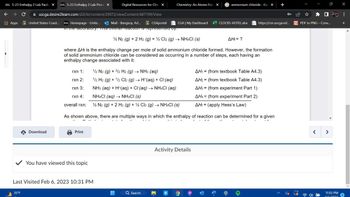
12 N2 (g) + 2 H2 (g) + 2Cl2 (g) → NH4Cl (s)
where AH is the enthalpy change per mole of solid ammonium chloride formed. However, the formation
of solid ammonium chloride can be considered as occurring in a number of steps, each having an
enthalpy change associated with it:
1/2 N2 (g) + 3/2 H2 (g) → NH3 (aq)
1/2 H2 (g) + 1/2 Cl2 (g) → H*(aq) + Cl(aq)
NH3 (aq) + H+ (aq) + Cl·(aq) → NH4Cl (aq)
NH4Cl (aq) → NH4Cl (s)
1/2 N2 (g) + 2 H2 (g) + ½ Cl2 (g) → NH4CI (S)
CGA | My Dashboard
Q Search
I.
As shown above, there are multiple ways in which the enthalpy of reaction can be determined for a given
Activity Details
81
ammonium chloride - Go X
CLOCKS-HOTEL.xlsx
2.
AH₁ = ?
d
https://cer.uscga.ed...
AH₁ = (from textbook Table A4.3)
AH₂ = (from textbook Table A4.3)
AH3 = (from experiment Part 1)
AH4 = (from experiment Part 2)
AH₁ = (apply Hess's Law)
+
I
PDF PDF to PNG - Conv...
PNG
11:03 PM
2/6/2022
X
![Jame
1 (1 pts) Initial Temperature of solution
2
Calculations! Certain questions will require you to show your work. Any question with an asterix (*) next to the point
value will require work to be shown. Label the work with what number problem you are doing.
3
4
(1 pts) Equation to calculate qwater (dsc'utior
is 1.0267 mL)
13
(1 pts) Final Temperature of solution
5 (3 pts)* Calculate qwater (in J)
6
(1 pts) Equation to determine moles from
molarity and volume
7 (3 pts) * Calculate moles of NH3
8
(1 pts) Equation to calculate AHxn using
moles and qwater
9 (3 pts) * Calculate Axn (in kJ/mol)
(2 pts)* Calculate ATwater
15
13 (1 pts) Initial Temperature of solution
11 (1 pts) Final Temperature of solution
12
(2 pts) * Calculate ATwater
(1 pts) Equation to calculate qwater (dsolution
is 1.0267 g/mL)
Part 1 (16 pts): Neutralization of aqueous ammonia (Atin Hs)
18
14 (3 pts)* Calculate qwater (in J)
16
(2 pts) Mass of NH4CI
17 (2 pts)* Calculate moles of NH4Cl
28 +3
(1 pts) Equation to determine moles from
mass and molar mass
(1 pts) Equation to calculate AHsol using
moles and qwater.
(3 pts) * Calculate AHsol (in kJ/mol)
H-C:
20 (2 pts) AH NH3 (ag) (Appendix Table A4.3)
21 (3 pts) AH, HCI (aq) (Appendix Table A4,3)
(Strong Acid so H+ (aq) + Ct (aq))
6.431
Enthalpy 2
mela
]-[91640) + (9014
apter 6: Energy and
Lab Title
46, 995 kJ/male
Part 2 (17 pts): Dissolution of ammonium chloride (AHsol, AH4)
23.00°C
28-47°C
M = 1
V
5.47°C
серь
q=merot qw = (Uw • disolution) Cs AT
MCSAT
2349.75 J
.05 maler
Анкхи = -ди
w/nR
22-09
14.97
-7.12
quz uw - dvolution) CUST
-3058.55 J
n = moles
n = m/M
m = mass
M = inilar mass
5-345 g
+ Matapb 0.1 male
J
A Hsol = - 9H₂0/ males
Андав
30585.5
Part 3 (5 pts): Hess's Law
80.3 kJ/mal
- 167.2
0.0
kJ / mal
HCl(aq) = -167-2
02
KJ/Ndci- (aq)
kJ mal
kJ / mal 7 H+ (aq) Page 3 of 8
Date
kJ/mal
x = -196 kJ
rxn
VU
KEY QUESTIONS
7. When are AH values equal to zero?
KJ/mol) + (1) (0 kJ/mol)] - [(2)(-18-
products & read](https://content.bartleby.com/qna-images/question/4d021649-84ae-494f-9309-8423bd446b81/57a9add6-4cb4-4d3d-bc87-687f4c62eca4/6f9yfqc_thumbnail.jpeg)
Transcribed Image Text:Jame
1 (1 pts) Initial Temperature of solution
2
Calculations! Certain questions will require you to show your work. Any question with an asterix (*) next to the point
value will require work to be shown. Label the work with what number problem you are doing.
3
4
(1 pts) Equation to calculate qwater (dsc'utior
is 1.0267 mL)
13
(1 pts) Final Temperature of solution
5 (3 pts)* Calculate qwater (in J)
6
(1 pts) Equation to determine moles from
molarity and volume
7 (3 pts) * Calculate moles of NH3
8
(1 pts) Equation to calculate AHxn using
moles and qwater
9 (3 pts) * Calculate Axn (in kJ/mol)
(2 pts)* Calculate ATwater
15
13 (1 pts) Initial Temperature of solution
11 (1 pts) Final Temperature of solution
12
(2 pts) * Calculate ATwater
(1 pts) Equation to calculate qwater (dsolution
is 1.0267 g/mL)
Part 1 (16 pts): Neutralization of aqueous ammonia (Atin Hs)
18
14 (3 pts)* Calculate qwater (in J)
16
(2 pts) Mass of NH4CI
17 (2 pts)* Calculate moles of NH4Cl
28 +3
(1 pts) Equation to determine moles from
mass and molar mass
(1 pts) Equation to calculate AHsol using
moles and qwater.
(3 pts) * Calculate AHsol (in kJ/mol)
H-C:
20 (2 pts) AH NH3 (ag) (Appendix Table A4.3)
21 (3 pts) AH, HCI (aq) (Appendix Table A4,3)
(Strong Acid so H+ (aq) + Ct (aq))
6.431
Enthalpy 2
mela
]-[91640) + (9014
apter 6: Energy and
Lab Title
46, 995 kJ/male
Part 2 (17 pts): Dissolution of ammonium chloride (AHsol, AH4)
23.00°C
28-47°C
M = 1
V
5.47°C
серь
q=merot qw = (Uw • disolution) Cs AT
MCSAT
2349.75 J
.05 maler
Анкхи = -ди
w/nR
22-09
14.97
-7.12
quz uw - dvolution) CUST
-3058.55 J
n = moles
n = m/M
m = mass
M = inilar mass
5-345 g
+ Matapb 0.1 male
J
A Hsol = - 9H₂0/ males
Андав
30585.5
Part 3 (5 pts): Hess's Law
80.3 kJ/mal
- 167.2
0.0
kJ / mal
HCl(aq) = -167-2
02
KJ/Ndci- (aq)
kJ mal
kJ / mal 7 H+ (aq) Page 3 of 8
Date
kJ/mal
x = -196 kJ
rxn
VU
KEY QUESTIONS
7. When are AH values equal to zero?
KJ/mol) + (1) (0 kJ/mol)] - [(2)(-18-
products & read
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For a certain chemical reaction ?H°= -35.4 kJ and ?S°= -85.5 J/K. Is the reaction endo or exothermic?arrow_forwardUsing the provided table and the equation below, determine the heat of formation for PbS. 2 PbS (s) + 3 02 (g) → 2 SO2 (g) + 2 PbO (s) AH° = -828.4 kJ/mol |kJ/mol Substance AH (kJ/mol) O2 (g) 1 2 3 SO2 (g) -296.9 4 6. C PbO (s) -217.3 7 8 9. +/- х 100 +arrow_forwardFor the reaction N₂ (g) + 3H₂(g) → 2NH3 (9) AGO = -40.9 kJ and AS atm. This reaction is at 258 K. = - 198.7 J/K at 258 K and 1 favored under standard conditions The standard enthalpy change for the reaction of 1.51 moles of N₂ (g) at this temperature would be kJ.arrow_forward
- 2. Use the standard reaction enthalpies given below to determine AH°rxn_for the following reaction: 2NO (g) + O2 (g) → 2NO2 (g); AH°xn = ? Given: N2 (g) + O2 (g) → 2NO (g) AH°rxn = 183 kJ ½ N2 (g) + O2 (g) → NO2 (g) AH°rxn = 33 kJarrow_forwardWhich equation represents the standard enthalpy change of formation of sodium chloride, AH;°? A. O Na(s)+ 0.5C|2(g) → NaCI(s) B. O 2Na(s) + Cl2(g) → 2NaC(s) C. O 2Na(l) + Cl2(g) → 2NaCI(s) D. O HCI(aq) + NaOH(aq) –→ NaCl(aq) + H2O(1)arrow_forward[References] The reusable booster rockets of the space shuttle use a mixture of aluminum and ammonium perchlorate as fuel. A possible reaction is 3A1(s) +3NH,CIo,(s) → Al,0,(s) + AlCls (s) + 3NO(g) + 6H2O(g) Calculate AH for this reaction. Substance and State AH; (kJ/mol) Al(s) Al2O3 (s) AICI3 (s) H20(g) NO(g) NH CIO.(s) -1676 -704 -242 90. -295 AH: kJ Submit Answer Try Another Version 1 item attempt remaining Prevarrow_forward
- Consider these reactions, where M represents a generic metal. 1. 2M(s) + 6HCl(aq) yields to 2MCl3(aq) + 3H2(g) Detal H1=-571.0 kJ 2. HCl(g) yields to HCl(aq) Delta H2=-74.8 kJ 3. H2(g) + Cl2(g) yields to 2HCl(g) Delta H3=-1845.0 kJ 4. MCl3(s) yields to MCl3(aq) Delta H4=-370.0 kJ Use the given information to determine the enthalpy of the reaction 2M(s) + 3Cl2(g) yields to 2MCl3(s) Use the correct number of significant digits.arrow_forwardPlease don't provide handwritten solution ....arrow_forward10. Calculate the standard enthalpy of formation of N₂O5 from the following data: kJ mol kJ 2NO(g) + O₂(g) →2NO₂(g) 4NO2(g) + O₂(g)→2N205(g) N₂(g) + O₂(g) →2NO(g) 4,H☺ = -114.1 = -110.2- 'mol kJ mol AH AH = + 180.5,arrow_forward
- A chemist measures the energy change AH during the following reaction: 2 NH,(9) - N,(9)+3H,(9) AH = 160. kJ Use the information to answer the following questions. O endothermic. This reaction is... alo O exothermic. Suppose 46.5 g of NH, react. O Yes, absorbed. O Yes, released. Will any heat be released or absorbed? O No. If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Be sure your answer has the correct number of significant digits.arrow_forwardUse the standard reaction enthalpies given below to determine AH° rxn 4 SO3(g) →4 S(s) + 602(g) AH°rxn = ? Given: SO2(g) →S(s) + O2(8) 25O2(g) + O2(g) →2SO3(g) 1583 kJ -494.6 kJ -791.4 kJ -293.0 kJ -692.4 kJ AH rxn = +296.8 kJ ΔΗ°, rxn = -197.8 kJ for the following reactionarrow_forwardA chemist measures the energy change AH during the following reaction: 2 HgO(s) → 2 Hg(1)+O,(g) AH=182. kJ Use the information to answer the following questions. endothermic. This reaction is... x10 exothermic. Yes, absorbed. Suppose 56.6g of HgO react. Yes, released. Will any heat be released or absorbed? No. If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. kJ Round your answer to 3 significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY