
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
1. Based on picture,
A. Classify the above boiler types based on technique
combustion and the position of the water flow!
B. Write a brief description of the 8 components in
steam power plant system above!
C. In addition to fuel, this power plant is also provided with
limestone and sand. What is the purpose of the two kinds of income
this compound?
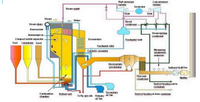
Transcribed Image Text:High pressire
turbine
Low pressure
turbine
Generator
Steam outlet
Gear
Gear
Steam
a Water
Dired
convderser
Steam dum
Downcomer
Ammonium in.
Compact solics separator.
Steamtrbine
condenser
Economizer
Feecwater tank
Fuel Limestone Sand
Feecwater inlet
Catalvtic converter
Meuring
equment,
emission
Electrostatic
precipittor
Indiced draftfan
Flue gas
condenser
Mffler
District heating out to
consumer
Secondary
Combustion
chamber
Bottom ash
District heating in from antemer
Tofly ash sib Primary air fan
air fan
Expert Solution
arrow_forward
Step 1
To answer based on the given picture
A. Classify the above boiler type based on technique combustion and the position of the water flow
B. Write a brief description of the 8 components in steam power plant system above
C. In addition to fuel, this power plant is also provided with limestone and sand. What is the purpose of doing so ?
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Calculate the performance ratio, specific heat transfer area, specific flow rate of cooling water, conversion ratio, and salinity of brine blowdown for a single stage flash desalination unit operating at the following conditions: Feed salinity = 45000 ppm Feed temperature = 25 °C Heating steam temperature = 90 °C Production capacity = 1 kg/s Brine blowdown temperature = 35 °C - - - - - Top brine temperature = 80 °C Terminal temperature difference in the condenser = 3 °C Thermodynamic losses = 2 °C.arrow_forwardChapter 6d BOOK: ENGINEERING THERMOFLUIDS, M. MASSOUD Data: Total loss coefficient = 2.5m^2 Flow area = 0.5m^2 Total rate of decay heat of DSC = 15kW System thermal length = 3.5m Air temperature that enters the HSM = 22C USE THE GIVEN DATA TO DETERMINE: 1. Temperature rise 2. Flow rate of air through the HSM 3. Total pressure drop from inlet to exitarrow_forward(optional) Design a solar collector: Following the steps to decide the size of a solar collector. Step1: the customer wants to heat 500 liters of water from 15 to 80 C. How much heat is required? Step2: the local daily solar insolation rate is 1.13*107 J/m2. How much heat the solar can provide per m2? Step3: if 33% of solar energy can be used to heat water (q) with a solar collector, how much heat can be used to warm up cold water? Step4: what collector surface area is A (m2) required?arrow_forward
- pls read the question carefully, i posted the same question more than one time but with different requirement. the subject name is Process plant design and safety, it's chemical engineering subjectarrow_forwardA _____________ reactor approaches a _____________ reactor at infinite recycle Fill in the blanks Choices. (a) completely mixed, plug-flow (b) plug-flow, completely mixed, (c) ideal, steadystate(d) plug-flow, idealarrow_forwardkindly answer this problem. draw the diagram. do not type answersarrow_forward
- P1arrow_forwardillustrates an endothermic reactor applied in the production of methanol. Design a P&ID for this reactor by considering that the reactor temperature and pressure are needed to be maintained at a specific value.arrow_forwardChemical Engineering Make a preliminary design for a separator to separate a mixture of steam and water;flow-rates: steam 2000 kg/h, water 1000 kg/h; operating pressure 4 bar.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The