
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
1. at what wavelength was Pb determined?
2. What is the slope of the calibration curve?
3. What is the y-intercept of the calibration curve?
4. The researcher determined the Pb content of water sample from a river suspected to be contaminated with Pb from a nearby factory by complexing with cyanidine and the absorbance measured was 0.405. What is the lead content of the water sample?
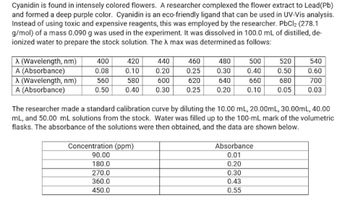
Transcribed Image Text:Cyanidin is found in intensely colored flowers. A researcher complexed the flower extract to Lead (Pb)
and formed a deep purple color. Cyanidin is an eco-friendly ligand that can be used in UV-Vis analysis.
Instead of using toxic and expensive reagents, this was employed by the researcher. PbCl2 (278.1
g/mol) of a mass 0.090 g was used in the experiment. It was dissolved in 100.0 mL of distilled, de-
ionized water to prepare the stock solution. The X max was determined as follows:
A (Wavelength, nm)
400
420
440
460
480
500
520
540
A (Absorbance)
0.08
0.10
0.20
0.25
0.30
0.40
0.50
0.60
A (Wavelength, nm)
560
580
600
620
640
660
680
700
A (Absorbance)
0.50
0.40
0.30
0.25
0.20
0.10
0.05
0.03
The researcher made a standard calibration curve by diluting the 10.00 mL, 20.00mL, 30.00mL, 40.00
mL, and 50.00 mL solutions from the stock. Water was filled up to the 100-mL mark of the volumetric
flasks. The absorbance of the solutions were then obtained, and the data are shown below.
Concentration (ppm)
90.00
Absorbance
0.01
180.0
0.20
270.0
0.30
360.0
0.43
450.0
0.55
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Substance P has an extinction coefficient of 72.22 mM-1cm-1 at 420nm. 2 μl of Substance P was added to 998 μl water and the Absorbance at 420nm was 0.820. Calculate the mM concentration of the original Substance P solutionarrow_forwardPLEASE ANSWER ASAParrow_forwardChemistry Instrumental Analysis Class The experiment is OES: Cleaning Validation of removal of pharmaceutical drugs and calibration standards by fluoresence spectroscopy - Why the instrument use two detectors and monochromators? What type of monochromators are used in our instrument? - Do you think that a similar experiment can be prepared for the analysis of naproxen and benzoic acid? Explain.arrow_forward
- The Beer’s Law plot of the standard curve for the equilibrium experiment was determined by plotting? absorbance versus [Fe3+] absorbance versus % transmittance % transmittance versus [FeSCN2+] absorbance versus [FeSCN2+]arrow_forwardMaria was tasked to determine the concentration of a Fe2(SO4)3 solution. She prepared 5 different standards of Fe2(SO4)3 using the table below as her guide. She ran the standards and the unknown solution through UV-VIS spectroscopy and recorded the absorbances of each solution. HINT: use the dilution equation to determine the concentration of Fe2(SO4)3 in each test tube before preparing the standard curve. NOTE: No need to force the line to pass through zero. Just graph the data as is. What is the slope of the line of the standard curve from the given data? What is the concentration of the unknown solution?arrow_forwardAn unknown amount of a compound with a molecular mass of 270.57 g/mol is dissolved in a 10 mL volumetric flask. A 1.00 mL aliquot of this solution is transferred to a 25 mL volumetric flask, and enough water is added to dilute to the mark. The absorbance of this diluted solution at 355 nm is 0.495 in a 1.000 cm cuvette. The molar absorptivity for this compound at 355 nm is €355 = 6149 M-¹cm-¹. What is the concentration of the compound in the cuvette? concentration: What is the concentration of the compound in the 10 ml flask? concentration: How many milligrams of compound were used to make the 10 mL solution? mass: M M mgarrow_forward
- B. Quantitative Spectroscopy Prepare two graphs, %T vs. Concentration and Absorbance vs. Concentration, using the data for the cobalt standards. The concentrations of Cobalt standards 2-4 can be calculated using simple dilution calculations. Concentration should be on the x-axis for each of these graphs. The first should clearly show some curvature while the second should be a linear curve. Using the absorbance graph you can determine the concentration of the unknown. Take the absorbance value for the unknown and find that value on the y-axis. Draw a horizontal line from that point on the y-axis to the plotted line. From the point where the horizontal line intersects the plotted line, draw a vertical line straight down to the x-axis. Record the value from the point where you hit the x-axis as the unknown concentration.arrow_forwardAbsorbance is not proportional to which of the following? a. Intensity b. Molar Absorptivity c. Concentration d. Path Lengtharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY