
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
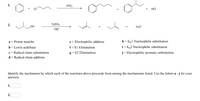
Transcribed Image Text:AICI,
HCI
2.
H2SO4
H20
HO"
130°
h = SN1 Nucleophilic substitution
i= Sy2 Nucleophilic substitution
a = Proton transfer
e = Electrophilic addition
b = Lewis acid/base
c = Radical chain substitution
f= El Elimination
g = E2 Elimination
j= Electrophilic aromatic substitution
d = Radical chain addition
Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - j for your
answers.
1.
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Na toluene reflux + NaCl .OH H₂O + 2. Br2 Br. HBr a = Proton transfer d = Radical chain addition g = E2 Elimination b = Lewis acid/base e = Electrophilic addition h = SN1 Nucleophilic substitution C = Radical chain substitution f = E1 Elimination i = SN2 Nucleophilic substitution Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. 1 2.arrow_forwardComplete the following reactions: indicate the mechanism (SN1 or SN2). If the reaction does not proceed, indicate so and the reason as to why it would not.arrow_forwarda = Proton transfer d = Electrophilic addition g = SN1 Nucleophilic substitution b = Lewis acid/base e = E1 Elimination h = SN2 Nucleophilic substitution c = Radical chain substitution f = E2 Elimination Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. 1.fill in the blank 1 2.fill in the blank 2arrow_forward
- Ph NME2 H2SO4 Ph OH Ph `NME2 NME2 Ph Ph Ph Tamoxifen anti (brest) cancer drug OH OH2 Br 2. HBr Proton transfer d = Electrophilic addition g = SN1 Nucleophilic substitution a b Lewis acid/base e = El Elimination h = SN2 Nucleophilic substitution c = Radical chain substitution f= E2 Elimination Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. 1 2. 1.arrow_forwardDraw structures and the mechanism for the organic products of the reaction below.arrow_forward1. Br Na NaBr Ph 2. CI NaOH но d = Radical chain addition g = E2 Elimination h = Sy1 Nucleophilic substitution a = Proton transfer b = Lewis acid/base e = Electrophilic addition c = Radical chain substitution f = E1 Elimination i = SN2 Nucleophilic substitution Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. 1. 2.arrow_forward
- How to attempt this problem?arrow_forwardComplete the Rxn schemesarrow_forwardThe following is a nucleophilic substitution reaction of R-3-chloro-2-methylhexane with "OCH3. The optical rotation of the solution after the reaction was complete was +11.4° CH3 Н. H3C. CH3 "OCH, H2 H. The mechanism for this reaction is SN2 Draw the organic molecule(s) which is(are) formed in this reaction. Do not include molecules like H,O or HCl. Draw the specific configuration (R or S) at any chiral carbons within your product(s). If both configurations are formed in the product(s), draw both as separate molecules/products.arrow_forward
- OH 1. H20 HI 2. BH3 O-BH3 d = El Elimination f = SN1 Nucleophilic substitution a = Proton transfer e = E2 Elimination g = SN2 Nucleophilic substitution b = Lewis acid/base c = Electrophilic addition The rections above involve synthesis or reactions of alcohols and ethers. Identify the mechanism by which they proceed from anmong the mechanisms listed. Use the letters a - g for your answers. 1. 2. Submit Answer Retry Entire Group 8 more group attempts remainingarrow_forward#16h. Provide the missing reactants, reagents, or products for the following reaction sequences below.arrow_forwardReview topics] [References) 1. CI NaOH NH2 NaCI H20 2. OH NAHCO3 12 H20 CO2 Nal a = Proton transfer d = Electrophilic addition g = SN1 Nucleophilic substitution b = Lewis acid/base c = Radical chain substitution e = El Elimination h = SN2 Nucleophilic substitution f= E2 Elimination Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. 1. 2. Retry Entire Group 9 more group attempts remaining nswerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY