
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
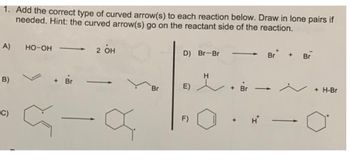
Transcribed Image Text:1. Add the correct type of curved arrow(s) to each reaction below. Draw in lone pairs if
needed. Hint: the curved arrow(s) go on the reactant side of the reaction.
2 он
A)
B)
C)
HO-OH
+ Br
a
Br
D) Br-Br
E)
F)
H
+ Br
Br
+ Br
+ H-Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I understand where the proton transfer occurs but not what the products will look like. Can you draw curved arrows to show proton transfer and what the reaction products would look like? And explain which side is favored and whyarrow_forwardPls help ASAParrow_forwardIn the reaction given below, what type of reaction is used?arrow_forward
- Draw the mechanism using curved arrows to show how the electron pairs move for the second step of the given reaction. Include lone pairs and formal charges (if applicable) on the structures. 2m H 哎 1-04 H + H₂O: "X" + HO: + H Harrow_forwardDon't use hand raiting pleasearrow_forwardI got step two wrongarrow_forward
- 2. a) Draw mechanism arrows to show how the reactants are converted to the products. b) Using the pKa values in Table 6.1, determine which side of the equilibrium will be favored. H-CI CląC Cl3C HO, H EH H Earrow_forwardClick on a basic (nucleophilic) atom. HH | | H-C-C=C₂ | H H H H-CI:arrow_forward4 -5 Draw the structure of the product that forms when the carbonyl compound shown is treated with K₂Cr₂O₂. If no reaction occurs, draw the structure of the organic starting material (reactant). COCH₂CH₂ Click and drag to start drawing a structure. 0 :0arrow_forward
- Consider the SN2 reaction between 1-bromo-2-methylpropane and methoxide. Add curved arrows to the starting materials to indicate the flow of electrons. Draw the product species to show the balanced equation, including nonbonding electrons and formal charges. H3C. C CH3 Draw curved arrows. H₂C H₂ C. / ||| ||| Select Draw Templates More Br: + :0 -CH3 CH₂ с Br O IU Br : 0 J Erase CH3 product Draw the products. The bromide ion has been drawn for you. Select Draw Templates More Br Erasearrow_forwardNeed to check answer 1.Borane (BH3) adds to alkenes to form an alkylborane. In the first box draw the mechanism arrows, and in the second box draw the correct product. Be sure to add lone pairs of electrons and nonzero formal charges to all species.arrow_forwardDon't use hand raiting pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY