
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
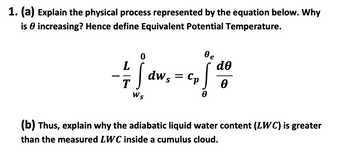
Transcribed Image Text:1. (a) Explain the physical process represented by the equation below. Why
is increasing? Hence define Equivalent Potential Temperature.
L
T
Ws
dw s =
Cp
0e
!
de
Ө
(b) Thus, explain why the adiabatic liquid water content (LWC) is greater
than the measured LWC inside a cumulus cloud.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Given: Propane, T = 18.8°C, h = 175 kJ/kg Find: u (kJ/kg) Your Answer:arrow_forwardI'm not sure but You may need to use Clausius-Clapeyron equation ,Whatever just do it correctly.arrow_forwardExercise 1 1: The specific volume of superheated water vapor at 0.6 MPa and 900°C is 0.90179 m³/kg in the steam tables, determine the specific volume also using: (a) The ideal-gas equation. :s) (b) The generalized compressibility chart. 'I (c) Can we consider the superheated vapor to behave as an ideal gas under the given temperature and pressure? Why?arrow_forward
- ts Suppose you have a gas that obeys the following modified van der Waals equation: P(V – nb) = nRT %3D a) In 1-2 sentences, provide a physical interpretation for this equation. HINT: Compare this with the octual van der Waais equation. What does the missing term represent? b) For a gas obeying this modified van der Waals equation, derive an expression for the work done by a reversible and isothermal change in volume. c) For this modified van der Waals equation (with n and b as constants), find the following two partial derivatives: i) ii) Tarrow_forwardQUESTIOΝ 1: A piston-cylinder arrangement contains 2 kg of steam originally at 200°C and x-0.9. The volume triples while thc temperature is held coustant. Pieten Calculate: (a) the linal pressure, in MPa, 7=200 C (c) the change in intermal energy, in kl.arrow_forwardPlease take Appropriate Values at the given conditions,Don't Copy.arrow_forward
- A cylinder fitted with a piston has a volume of 0.1 m'and contains a 0.8 kg of steam at 0,.4 MPa. Heat is trausferred to the steam umntil the temperature is 250°C, while the pressure remmains constant. (1) vi (specific volume) in m'kg = (2) v: (specific volume) in m'kg = (3) xi (quality) = . (4) us (specific internal energy) in k/kg (5) uz (specific internal energy) in kJ/kg =. (6) Xx: (quality)= (7) Phase description at imitial state is. (8) Phase description at fitsal state is (9) W (work) in kJ= (10) Q (heat) in kJ =arrow_forwardi need the answer quicklyarrow_forwardAs shown in the figure, a tank fitted with an electrical resistor of negligible mass holds 2 kg of nitrogen (N₂), initially at 27°C, 0.1 MPa. Over a period of 10 minutes, electric power Eelectric of 0.35 kW is provided to the resistor. During this same period, a heat transfer of magnitude 10.5 kJ occurs from the nitrogen to its surroundings. Assume ideal gas behavior, but do not assume constant specific heats. Determine the final temperature, in °C, and the final pressure, in MPa. Nitrogen, N₂ m = 2 kg T₁ = 27°C P₁ = 0.1 MPa eeeeeee Qout, 12 = 10.5 kJ É electric At 10 minutesarrow_forward
- Given: Water, T = 103.6°F, u = 961.5 Btu/lbm %3D Find: quality, x (%) Your Answer:arrow_forwardThe expression for the Joule Thompson coefficient for a gas described by the van der Waals equation of state, at low pressures, can be expressed as: 1 2a -—- (2017-b) Cp\R* T i) What will μT be for an ideal gas? Why must this be the case? ii) How does this expression simplify at high temperature? a) Will μT be positive or negative? b) How does temperature change under conditions of low pressure and high temperature, when this gas expands? iii) How does this expression simplify at low temperature? a) Will μT be positive or negative? b) How does temperature change under conditions of low pressure and low temperature, when this gas expands? iv) Calculate the Joule Thompson coefficient of ethane at 300K and 600K. HJT = You can find the heat capacity needed at the NIST Webbook. Search for ethane and then go to the "Gas phase thermochemistry data." Use the values for 1 bar. Pay close attention to units. Your answer should have units of K/atm. The vdw parameters for ethane are: a…arrow_forwardI need the answer as soon as possiblearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY