
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
what is the experimental and theoretical yield of CuCl? Make sure you have the correct sig figs.
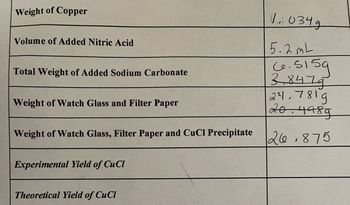
Transcribed Image Text:**Weight of Copper:** 1.034 g
**Volume of Added Nitric Acid:** 5.2 mL
**Total Weight of Added Sodium Carbonate:**
- 6.515 g
- 3.847 g
**Weight of Watch Glass and Filter Paper:**
- 21.781 g
- 20.498 g
**Weight of Watch Glass, Filter Paper and CuCl Precipitate:** 26.875
**Experimental Yield of CuCl:** *(No value provided)*
**Theoretical Yield of CuCl:** *(No value provided)*
**Explanation:**
This table lists measurements used in a chemical experiment involving the reaction of copper and other substances. The weights and volumes are noted for the purpose of calculating both experimental and theoretical yields of CuCl. Specific weights are provided for copper, nitric acid, sodium carbonate, and the materials used in measuring the precipitate.
Expert Solution
arrow_forward
Step 1
According to the question we have,
The weight of copper (Cu) is given by = 1.034 gm
The volume of nitric acid (HNO3) added is given by = 5.2 ml
The total weight of the sodium carbonate added is given by = 6.515 gm
The weight of the watch glass and filter paper is given by = = 24.781 gm
The weight of the watch glass, filter paper, and CuCl precipitate is given by = =26.875 gm
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the Haber-Bosch process for the synthesis of ammonia from its elements. Calculate the theoretical yield in moles NH3 from the complete reaction of 59.8 grams N2 in the presence of excess H2 gas according to the following balanced chemical equation: N2(g) + H2(g) → NH3(g) X TING AMOUNT ADD FACTOR ANSWER RESET *( ) 17.04 3.52 1.01 14.01 59.8 3 4.27 8.54 72.7 2.13 28.02 145 1 7.04 g/mol N2 g N2 g/mol NH3 mol NH3 mol N2 g NH3arrow_forwardCalculate the mass, in grams, of 322 atoms of iron, Fe (1 mol of Fe has a mass of 55.85 g). 1 4. 8. +/- 2.arrow_forwardsomeone help with this question!arrow_forward
- D M [Review Topics] [References] Use the References to access important values if needed for this question. For the following reaction, 5.54 grams of oxygen gas are mixed with excess carbon monoxide. The reaction yields 12.1 grams of carbon dioxide. 2CO(g) + O2(g) → 2CO2(g) a. What is the theoretical yield of carbon dioxide? g b. What is the percent yield for this reaction? % Submit Answer Retry Entire Group 6 more group attempts remaining Cengage Learning | Cengage Technical Support Previous Next> Save and Exitarrow_forwardGaseous methane (CH4) reacts with gaseous oxygen gas (0₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). What is the theoretical yield of carbon dioxide formed from the reaction of 0.80 g of methane and 2.1 g of oxygen gas? Round your answer to 2 significant figures. THED X 5 ? Explanation Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility DII DD J 4) F1 F8 F9 F10 F12 T 0 esc ! 1 @ 2 Check F2 #3 80 F3 S4 $ 000 000 F4 % 5 F5 MacBook Air F6 A 6 & 7 44 F7 * 00 8 ( 9 - F11 + 11 A alo Ar 00 delearrow_forwardConsider the following chemical reaction HCl (aq) + HgNO3 (aq) à Hg2Cl2 (s) + HNO3 (aq) If 20.0 grams of HCl is mixed with 30.0 grams of HgNO3 Determine the excess reactant The amount of the solid precipitate that was recovered equals to .................. grams , if the percentage yield from this reaction was determined to be 78.4%. Write the net ionic equation for the chemical reaction Determine the type of this chemical reactionarrow_forward
- Liquid octane CH3CH26CH3 reacts with gaseous oxygen gas O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O. If 0.689g of water is produced from the reaction of 1.1g of octane and 2.1g of oxygen gas, calculate the percent yield of water. Round your answer to 2 significant figures.arrow_forwardA 4.86 g quantity of solid rubidiummetal is combined with 3.44 g of magnesium chloride crystals according to this chemical reaction: 2 Rb (s) + 1 MgCl2 (s) 1Mg (s) +2 RbCI (s) What quantity of magnesium metal will be produced? _g Mg round your answer using appropriate sig figs The following molar masses may or may not be needed, but are provided to save you time: • molar mass: Rb = 85.47 g/mol • molar mass: MgCl2= 95.21 g/mol • molar mass: Mg = 24.31 g/mol Hiint: Start with the number of grams of Rubidiumarrow_forwardThe actual yield of one of the reactions in 43.5g. What was theoretical yield in the laboratory if the scientist concluded that % yield of the reaction ended up to be 77%.arrow_forward
- (0) Mass of FeCl2 (g) Volume of KMnO4(mL) Molarity of KMnO4 Trial 1 1.9986 33.81 0.0185 Trial 2 2.0237 32.33 0.0185 Trial 3 2.002 33.58 0.0185 The molecular weight of FeCl2 is 151.91 g/mol. In this assignment, you will determine the mass % of an unknown sample of ferrous chloride (FeCl2) by titrating it with a KMnO4 solution of known concentration. 11. What is the average % iron in the unknown sample using your best three answers?arrow_forwardThe theoritical yeild of a certain compound according to a chemical reaction is 127 g. The percent yield is 82.0%. When the reaction is carried out, what is the actual yeild?arrow_forward1) The Law of Conservation of Mass states that the total mass, in an open system, does not change as the result of reactions between its parts. True False 2) The deviation from the theoretical yield to the actual yield is called the percent yield. True Falsearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY