
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
1) Please list all glycosidic linkages between each monosaccharide units. For example, α(1→4)
2) Please discuss whether these oligo/polysaccharides would be reducing or non-reducing sugar. Remember to state your reasoning in complete sentence.
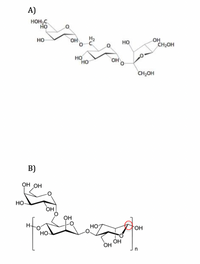
Transcribed Image Text:### Carbohydrate Structures
The image presents two chemical structures of carbohydrates, labeled as A and B.
#### A) Structure
This structure represents a disaccharide known as sucrose, which is composed of two monosaccharides: glucose and fructose. The structure is characterized by:
- The glucose unit (left) is linked to the fructose unit (right) via a glycosidic bond.
- Hydroxyl (OH) groups are attached to several carbon atoms in both monosaccharides.
- The glycosidic bond involves the 1-carbon of glucose and the 2-carbon of fructose.
#### B) Structure
This structure illustrates a polysaccharide, specifically cellulose, with multiple glucose units. Key features include:
- The linear arrangement of β-D-glucose units indicates a long chain.
- The glycosidic linkages are β(1→4), which connect the glucose molecules, highlighted by the circled area.
- Hydroxyl (OH) groups are present on each glucose unit, contributing to hydrogen bonding.
- The repeating unit is denoted by the bracket with the variable "n" indicating the polymer chain can have multiple repeating glucose units.
These structures highlight the diversity and complexity of carbohydrate molecules, ranging from simple to complex polysaccharides, playing essential roles in energy storage and structural integrity in living organisms.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- What are the glycosidic linkages of the trisaccharide shown? α1→6, α1→6 α1→6, β1→6 α1→6, β1→5 α1→6, α1→5arrow_forwardThe structure below shows that of a trisaccharide that is composed of (going from top left to bottom right) galactose, glucose, and fructose. More specifically, the component monosaccharides are [D-galactose, L-galactose], [D-glucose, L-glucose], and [D-fructose, L-fructose] .arrow_forwardquestion 1 : draw an α,α(2, 2) linkage between the two monosaccharides(two fructose ). label the glycosidic linkage. Must be Haworth question 2 : Draw glycosidic linkage between D-glucose linked to D-altrose with a β(1, 3) glycosidic linkage. label clearly the glycosidic linkage. must be Haworth.arrow_forward
- Consider the disaccharide maltose. сон H -= H OH OH Part 1 of 2 д- CH₂OH H H H он Н Н д- ОН Н Он Н Он Classify the glycosidic linkage as a or ẞ. Select the single best answer. α В Part: 1/2 Part 2 of 2 Modify the structure of maltose to show the monosaccharide products that form from hydrolysis. You may draw the structures in any arrangement th you like, so long as they aren't touching. Н он Снон Н -= Он Н снон Н H H Он Н д- Н он -= Н Он H Онarrow_forwardYour friend has discovered a protein that they suspect is glycosylated. They decide to perform a series of tests to determine the nature of the oligosaccharide. Assuming/Knowing the following: Fucose molecular weight is approximately 164 Galactose molecular weight is approximately 180 GalNAc molecular weight is approximately 221 Mannose molecular weight is approximately 180 Sialic Acid molecular weight is approximately 309 Neuraminidase cleaves before a Sialic Acid (Sialic Acid and anything after leaves the protein) Beta-galactosidase cleaves after a Galactose (Galactose and anything before remains on the protein) Peanut Agglutinin binds to GalNAc Concanavalin A binds to Mannose Hemagglutinin binds to Sialic Acid Based on preliminary results, they suspect that the oligosaccharide is 7 glycosides long and weighs a total of 1414 g/mol They carried out the following experiments: they treated the protein with either a glycosidase or a lectin, and then pelleted the protein using…arrow_forward2) Consider the following disaccharide which was formed from the breakdown of a polysaccharide found in plant material. ОН Glycosidic linkage: H H OH H H НО H H. OH H. ОН OH H НО H ОН a. Label the glycosidic linkage above in the following format: a/B(# → #) b. Would you expect this disaccharide to have been formed from amylose or amylopectin? c. Describe at least two chemical tests that would give insight into the structure of this disaccharide, assuming you've isolated it and wanted to elucidate the structure from scratch. Include detail on not only the tests you would use but what results you would expect to get given the structure above. Assume you can analyze the structure of any monosaccharide you get from your chemical tests.arrow_forward
- Consider the disaccharide trehalulose. CH₂OH H Н Он H он Н он H CH2 HO Н Н носн,он Он Н Part: 0/3 Part 1 of 3 Highlight the glycosidic linkage in the structure of trehalulose. Снон Н H он Н он Н Он Н CH2 но Н H HỌ/CH,OH Он H ✓arrow_forwardDraw both possible disaccharides of D-psicose and D-mannose that are joined by an α(1→4) glycosidic linkage. Are either of them now a reducing sugar?arrow_forwardModify the mannose structures provided to show the structure of a disaccharide formed from two mannose units joined by a 1 → 6-B-glycosidic linkage. CH₂OH Снон H ОН H Он Н H Он он OH Он Он H он Н H H H H Х Garrow_forward
- In order to metabolize lactose, most infants express the enzyme lactase in their intestines. What kind of glycosidic linkage does lactose have? Please draw the individual monosaccharides to explain your answer. Please explain how the monosaccharides in lactose will be used in glycolysis. Be specific.arrow_forwardThe human body uses the branched polymer glycogen for short term storage of glucose in the liver. It is broken down by the stepwise removal of the terminal glucose monomer. Explain why it is an advantage for glycogen to have a branch chain structure rather than a linear structure. (A pargraph would be great)arrow_forwardWhich of the following statements is correct regarding the structures below? CHO CHO CHO -Н НО H НО н- ОН -Н - ОН CH2OH CAB All are structures of D-sugars. All are structures of ketohexoses. Only CAR is a monosaccharide. O Only CAP is a L-sugar. -ОН -ОН H-OH H-OH H- ОН CH₂OH CAR но-н H-OH н НО т н-он CH₂OH CAP CH₂OH Fo НО-Н H НО-Н H он CH₂OH CATarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON