
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
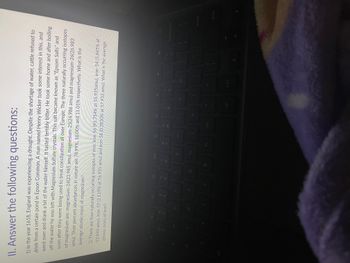
Transcribed Image Text:**II. Answer the following questions:**
1) In the year 1618, England was experiencing a drought. Despite the shortage of water, cattle refused to drink from a certain pond in Epsom Common. A man named Henry Wicker took some interest in this, and went over and drank a bit of the water himself. It tasted terribly bitter. He took some home and after boiling off the water he was left with Magnesium Sulfate crystals. This salt became known as "Epsom Salts" and soon after they were being used to treat constipation all over Europe. The three naturally occurring isotopes of magnesium are: magnesium-24 (23.985 amu), magnesium-25 (24.986 amu), and magnesium-26 (25.983 amu). Their percent abundances in nature are 78.99%, 10.00%, and 11.01% respectively. What is the average atomic mass of magnesium?
2) There are four naturally occurring isotopes of iron: iron-56 (91.754% at 55.935 amu), iron-54 (5.845% at 53.940 amu), iron-57 (2.119% at 56.935 amu) and iron-58 (0.2830% at 57.933 amu). What is the average atomic mass of iron?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Suppose you added 40 mL of water to your vinegar sample instead of 20 mL. Would the titration have required more, less or the same amount of NaOH(aq) for a complete reaction? Explain.arrow_forwardA 25.00 mL sample of 0.125 M sulfuric acid solution is titrated with 0.500 M sodium hydroxide solution. Write the balanced chemical equation for the neutralization reaction.arrow_forwardH2. Titrations are performed by a scientist in the laboratory as follows: a solution of deuterated hydrochloric acid contains 1.08 g DCl in 750 ml of deionised, distilled water. An average amount of 14.95 ml of the DCl(aq) solution is titrated with 25.0 ml of Ca(OH)2 to produce an endpoint (as determined by an appropriate indicator). What is the molarity of the Ca(OH)2 solution? You must include all of your calculations Please give typed answerarrow_forward
- An analytical chemist weighs out 0.193 g of an unknown diprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. She then titrates this solution with 0.1200 M NaOH solution. When the titration reaches the equivalence point, the chemist finds she has added 24.0 mL of NaOH solution. Calculate the molar mass of the unknown acid. Be sure your answer has the correct number of significant digits. g mol x10 X Śarrow_forwardAn analytical chemist weighs out 0.181 g of an unknown diprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. She then titrates this solution with 0.1700 M NaOH solution. When the titration reaches the equivalence point, the chemist finds she has added 15.9 mL of NaOH solution. Calculate the molar mass of the unknown acid. Be sure your answer has the correct number of significant digits. ol. g mol Ararrow_forwardBy titration it is found that 35.7 mL of 0.101 M NaOH(aq) is needed to neutralize 25.0 mL of HCl(aq). Calculate the concentration of the HCl solution.arrow_forward
- By titration, it is found that 19.1 mL of 0.157 M NaOH(aq) is needed to neutralize 25.0 mL of HCl(aq). Calculate the concentration of the HCI solution.arrow_forwardI have 28.63 mL of an unknown-concentration Mg(OH)2 (aq) solution. I find that, when I add 23.33 mL of 0.1060 molar HCl(aq) to this solution, the acid and base exactly neutralize each other. What was the original concentration of the Mg(OH)2 solution? a. 3.151 molar O b. 0.1728 molar c. 0.04319 molar d. 0.06504 molar e. 0.08638 molar Clear my choice Previous page Z 3 $ 4 n 5 80 MacBook Pro 6 & 11 8 Next pagearrow_forwardA 1.22 g sample of KHP is used to titrate a NaOH solution. A student finds that it requires 15.00 mL of the NaOH solution to reach the end point. What is the molarity of the NaOH solution?arrow_forward
- By titration, it is found that 39.1 mL of 0.161 M NaOH(aq) is needed to neutralize 25.0 mL of HCl(aq). Calculate the concentration of the HCl solution.arrow_forwardA chemistry student weighs out 0.0852 g of phosphoric acid (H,PO,), a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1500 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Be sure your answer has the correct number of significant digits. mLarrow_forwardThe U.S. standard for arsenate in drinking water requires thatpublic water supplies must contain no greater than 10 partsper billion (ppb) arsenic. If this arsenic is present as arsenate,AsO43-, what mass of sodium arsenate would be present ina 1.00-L sample of drinking water that just meets the standard?Parts per billion is defined on a mass basis asppb =g soluteg solution * 109arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY