
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Can you please help with the following
Provide the mechanism involved in the reaction and what the major product(s) would be.
Thank you
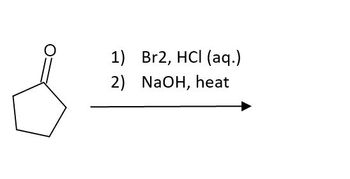
Transcribed Image Text:1) Br2, HCl (aq.)
2) NaOH, heat
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the major organic product formed when the compund shown below undergoes a reaction with H2O2 and then is heated in H2O. The 2nd photo is what I thought was the answer, but when I put it into Mastering Chemistry, it was counted as wrong and my feedback was, "What configuration is the C=C bond expected to haev?" I'm confused as to what this is referring to, so I appreciate any help given.arrow_forwardPlease help me with the organic chemistry question below. Please share details on each step to aid in understanding. Thank you.arrow_forwardOrganic chemistry student Everett has set up two separate electrophilic addition reactions with 2-butyne (shown below). In reaction (i) the alkyne is treated with 1 equivalent of HBr, which successful provides (Z)-2-bromo-2-butene. In reaction (ii) the alkyne is subjected to a two-step hydroboroation/oxidation sequence. Interestingly, Everett’s second reaction fails to provide the desired methyl ketone and instead has provided butan-2,3-diol. (a) Provide the structure of intermediate X form the first step of reaction (ii). (b) Briefly explain (use structures if necessary) why reaction (i) provided an alkene where as reaction (ii) provided an alkane when only 1 equivalent of the electrophile was used in each reaction.arrow_forward
- Identify the electrophile in the following electrophilic addition reaction step. Explain your choice.arrow_forwardShow the mechanism of the reaction of drawing 1 mole of water from the 2,2,5-trimethyl-3-hexanol compound, indicating the reaction conditions, step by step. Indicate the main product and by-product. b) Does the main product show the geometric isomer? If it does not show the isomers, please indicate why it does not. c) Write the products formed when the. main product ozonlamp is reduced.arrow_forward3) Fill in the red box(es) with the missing reactant(s), reagent(s), product(s), solvent, and/or conditions and write a reasonable mechanism for the reaction. Not every box needs to be used and not every box necessarily corresponds to only a single species. If no reaction occurs OR no reagents exist to perform the reaction state, "NOT POSSIBLE" AND explain why. Polymer HBrarrow_forward
- What is/are the product(s) of the reaction of 2-bromopropane with NaOCOCH3? Draw the mechanism & identify it as Sn1, Sn2, E1, or E2. Please explain your reasoning.arrow_forwardPlease answer with All the required steps needed to solve itarrow_forwardplease explain all steps, I am having trouble grasping this process :)arrow_forward
- Please help with thisarrow_forwardPlease help me with this question, it's urgent The reaction shown below does not follow the expected theory. Given what you learned about substitution/elimination, what is the expected mechanism this reaction should follow? Suggest why that is not what happens and give the actual product of the reaction.arrow_forwardPlease don't provide handwritten solution ....arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY