
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
infoPractice Pack
Question
infoPractice Pack
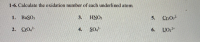
Transcribed Image Text:1-6. Calculate the oxidation number of each underlined atom.
1. BaSO3
3.
HNO:
2. Сю.
CrO.
4.
SO-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Includes step-by-step video
Trending nowThis is a popular solution!
Learn your wayIncludes step-by-step video
Step by stepSolved in 5 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- C What volume in milliliters of 0.0130 M Ca(OH)2 is required to neutralize 70.0 mL of 0.0300 M HBr? 17 ! F1 1 @ 2 9,841 F2 #3 # 3 80 F3 JUN 23 S4 $ 4 O Q F4 átv 5 2 Question 1 of 10 F5 MacBook Air A 6 G F6arrow_forwardRépondre au questionnaire 1. A metal cylinder has a mass of 276.36 grams and the measurements shown below. diameter 25.68 mm length = 152.45 mm Calculate the density of the metal cylinder in g/mL. diameter = 2(radius) and Vcylinder p(radius)²(length) 2. Write the Balanced Complete Molecular and the Net Ionic Equations for the reaction of Aqueous Silver (1) Nitrate with Aqueous Sodium Carbonate. (show all states) Sortir 3. The solubility of KNO3 in water at 0 °C is 13.3 grams per 100 mL of water. If I add 20.0 grams of KNO3 to 30.0 mL of water at 0 °C, how many grams of undissolved solid KNO3 should there be?arrow_forward1. Reaction 1: 4Ag(s) + 2H2S(g) + O2(g) + 2Ag2S(s) + 2H₂O(g) a. Assign oxidation states to all elements in both reactants and products in Rxn 1. b. Which species is being oxidized? Which species is being reduced? c. Which species is the oxidizing agent? Which species is the reducing agent?arrow_forward
- s) 0.250 g of HI is dissolved in 300. mL of a 0.00520 M solution of Ca(OH)2. (Assume this does not change the volume). The dissolved salts then proceed to react. a. What is the balanced chemical reaction (include states)? b. What type of reaction is this? С. What is the limiting reactant? d. How many grams of water are produced from this reaction?arrow_forward11. Complete and balance (if necessary) the following neutralization reaction. Include state symbols! HBr(aq) + KOH(aq) --> b. Name the products. c. Write the complete ionic and net ionic equations for this reaction.arrow_forwardClassify the following type of reaction: AgNO3(aq) + NaCl(aq) ----> NaNO3(aq) + AgCl(s) a. Neutralization b. Single Displacement c. Double Displacement d. Neutralizationarrow_forward
- Determine whether each compound is soluble or insoluble. Forthe soluble compounds, list the ions present in solution.a. AgI b. Cu3(PO4)2 c. CoCO3 d. K3PO4arrow_forward1. What is the mass of the solution when 50.0 mL of 1.0 M HCI solution is mixed with 50.0 mL of 1.0 M NaOH solution? (d = 1.02 g/mL )arrow_forwardWhat volume of 0.0521 M Ba(OH)2 is required to neutralize exactly 14.20 mL of 0.141 M HC1? a. 95.2 mL Ob. 19.2 mL OC. 191 mL Od. 38.4 mLarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY