
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
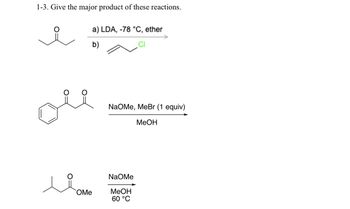
Transcribed Image Text:1-3. Give the major product of these reactions.
a) LDA, -78 °C, ether
b)
NaOMe, MeBr (1 equiv)
MeOH
OMe
NaOMe
MeOH
60 °C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Do the reactions below proceed in good yield from left to right as shown? a) + H₂O OH ΝΗΣ b) excess OH + trace of Na OCH2CH3 OH J. I OH + H₂N-CH3arrow_forwardComplete the reaction map by providing the major product. #2 E SOCI # 1 E- OH CH3CI KMnO4 - А PDC B O 2 -NH₂ OH OH PCC A D LIAIH4 H* MgCl H* B HCI Carrow_forwardPredict the major product for the following reaction. NaOHH,0 H. он OH H. H. HO, II III I H. HO IV V 1) I 2) || O 3) II O 4) IV O 5) varrow_forward
- I want question number 8 but the C part from question number 7 abovearrow_forwardProvide the major organic product of the reaction shown. NV L Draw the molecule on the canyas by choosing buttons from the Tools (for bonds), t [1] A OH 4' 1. NaH эсе 2. CH₂CH₂CH₂CH₂I H: 120 EXP. CONT L Marvin JS ? ** I U ZOS CI Brarrow_forward18 and 20 only thank uarrow_forward
- Phenol plus 1) HNO3 H2SO4: 2) Br2 + AICI3; 3) 1/2 l2, HNO3. can yield O 1-bromo-2-iodo-3-nitrophen-4-ol O 2-bromo-3-iodo-4-hydroxy-nitrobenzene O 3-bromo-5-iodo-4-nitrophenol 1-bromo-3-iodo--4-hydroxy-nitrobenzene O 2-bromo-6-iodo-4-nitrophenolarrow_forwardWhich of the following reactions will not generate an alcohol group in the product? (1) CH,CH;MgBr Reaction A (2) dil. H* /H;O NaOH Br Reaction B H2O//THF (1) Hg(OAc)/ H0 Reaction C (2) NABH/ethanol NAOH Reaction D H2O//THF OReaction B Reaction A Reaction C Reaction Darrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY