Question
The
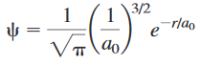
Transcribed Image Text:1
1
3/2
-rlao
e
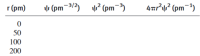
Transcribed Image Text:r (pm)
v (pm-3/3)
? (pm-3)
4aP? (pm¬1)
50
100
200
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps

Knowledge Booster
Similar questions
- Please asaparrow_forwardNeeds Complete typed solution with 100 % accuracy.arrow_forwardVac uum → measure the Electric Rield Oue wave lenglat away Ampli tudo is 2u V/m At 2=2 em >And Find : the frequency write the expression Ahe descvi bes the slectric peld if it is traaling positive Ź divection in time demain Find Harrow_forward