
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
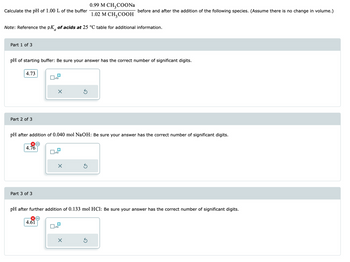
Transcribed Image Text:Calculate the pH of 1.00 L of the buffer
Part 1 of 3
Note: Reference the pK of acids at 25 °C table for additional information.
a
4.73
pH of starting buffer: Be sure your answer has the correct number of significant digits.
Part 2 of 3
×...
4.76
Part 3 of 3
x10
×
pH after addition of 0.040 mol NaOH: Be sure your answer has the correct number of significant digits.
4.61
x10
X
Ś
x10
0.99 M CH3COONa
1.02 M CH₂COOH
X
before and after the addition of the following species. (Assume there is no change in volume.)
Ś
pH after further addition of 0.133 mol HCl: Be sure your answer has the correct number of significant digits.
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- O ACIDS AND BASES Calculating the pH of a buffer A solution is prepared at 25 °c that is initially 0.077M in trimethylamine ((CH3)3N), a weak base with K₁=7.4× 10-4, and 0.50M in trimethylammonium Calculate the pH of the solution. Round your answer chloride ((CH3), NHCI) to 2 decimal places. pH = 1/3 Xarrow_forwardAcetic acid has a Ka of 1.8 x 10¬º. Three acetic acid/acetate buffer solutions, A, B, and C, were made using varying concentrations: A. [acetic acid] ten times greater than [acetate], B. [acetate] ten times greater than [acetic acid], and C. [acetate] = [acetic acid]. Match each buffer to the expected pH. Drag the appropriate items to their respective bins. • View Available Hint(s) Reset Help [acetate] ten times greater than [acetic acid] [acetate] = [acetic acid] [acetic acid] ten times greater than [acetate] pH = 3.74 pH = 4.74 pH = 5.74arrow_forwardWhat is the [H3O+] and the pH of a benzoic acid-benzoate buffer that consists of 0.13 M C6H5COOH and 0.30 M C6H5COONa? (Ka of benzoic acid = 6.3 × 10−5) Be sure to report your answer to the correct number of significant figures. [H3O+] = ___× 10___MpH =____arrow_forward
- A 1.451.45 L buffer solution consists of 0.1590.159 M butanoic acid and 0.3320.332 M sodium butanoate. Calculate the pH of the solution following the addition of 0.0620.062 moles of NaOHNaOH. Assume that any contribution of the NaOHNaOH to the volume of the solution is negligible. The ?aKa of butanoic acid is 1.52×10−51.52×10−5.arrow_forwardWhat is the pH of a buffer that contains 0.356 M H2C6H606 and 0.647 М НС,Н,О6? Ka1 = 7.9 × 10-5 Ka2 = 1.6 x 10-12 IMPORTANT: When entering your answer: • Erter the number only (no units) • Do not leave any spaces • Use a leading zero before the decimal when necessary Report your number to 2 decimal places (regardless of the significant figures) • Correctly round your answer to the 2nd decimal place (allowed margin of error is only + 0.01, so always use un- rounded numbers in your calculations) Examples: 0.12 or 9.87arrow_forwardPlease don't provide handwritten solution... A 1.0 L solution contains 0.100 mol HC2H3O2 and 0.100 mol NaC2H3O2. The value for Ka HC2H3O2 is 1.8 X 10-5. What is the pH of the buffer after adding 0.010 mol NaOH to the buffer.arrow_forward
- A chemist titrates 100.0 mL of a 0.3992 M ethylamine (C,H;NH,) solution with 0.6385 M HCl solution at 25 °C. Calculate the pH at equivalence. The p K, of ethylamine is 3.19. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HCl solution added. pH =arrow_forwardNonearrow_forwardBuffer capacity is a measure of a buffer solution's resistance to changes in pH as strong acid or base is added. Suppose that you have 105 mL of a buffer that is 0.260 M in both benzoic acid (CH₂COOH) and its conjugate base (CH₂COO¯). Calculate the maximum volume of 0.270M HCl that can be added to the buffer before its buffering capacity is lost. volume: mLarrow_forward
- A 10.0mL solution of 0.720 M NH3 is titrated with a 0.240 M HCl solution. Calculate the pH after the following additions of the HCl solution: 0.00mL, 10.0mL, 30.0mL, and 40.0mL. Be sure your answer has the correct number of significant digits. 0.00 mL added, pH = 10.0 mL added, pH = 30.0 mL added, pH = 40.0 mL added, pH =arrow_forwardPart 3: pH after addition of 0.145mol HCl: Round your answer to 2 decimal placesarrow_forwardA chemist titrates 90.0 mL of a 0.2182M acetic acid (HCH3CO₂) solution with 0.8569M NaOH solution at 25 °C. Calculate the pH at equivalence. The p K of acetic acid is 4.76. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of NaOH solution added. pH = 0 X 3 ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY