
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
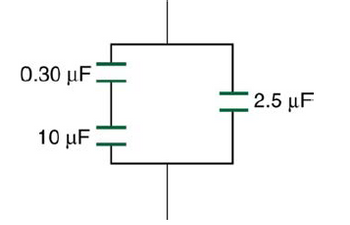
Transcribed Image Text:0.30 uF
10 uF
th
2.5 uF
Expert Solution
arrow_forward
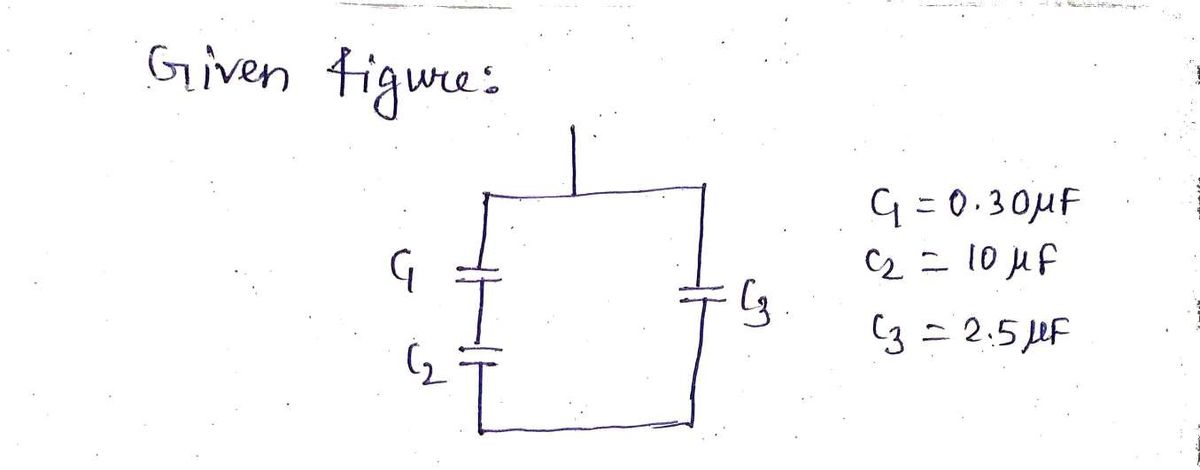
Step 1: Given
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- #3arrow_forward1. A walrus transfers energy by conduction through its blubber at the rate of 165 W when immersed in -1 deg C water. The walrus's internal core temperature is 36.5 deg C, and it has a surface area of 2 m². What is the average thickness of its blubber, which has the conductivity of fatty tissues without blood (k=0.2 J/(s m °C))? d= cmarrow_forwardIf 65.0L of oxygen at 20.0°C and an absolute pressure of 2.75atm are Compressed to 49.2L and at the Same time the tempurature is raised to S3.5°C, what will the pressure be? P= atmarrow_forward
- 2. Show that if the temperature on the Celsius scale changes by ATc, the Fahrenheit temperature changes by AT, = {ATc. 3. A solid substance has a density po at a temperature To. If its temperature is increased by an amount TA, show that its density at the higher temperature is given by Po 1+ BAT 4. A circular copper ring at 20.0°C has a hole with an area of 9.980 cm². (a) What minimum temperature must it have so that it can be slipped onto a steel rod having SOarrow_forward2. A spherical balloon is made from a material whose mass is 3.30 kg. The thickness of the material is negligible compared to the 1.45 m radius of the balloon. The balloon is filled with helium (He) at a temperature of 295 K and just floats in air, neither rising nor falling. The density of the surrounding air is 1.19 kg/m3 and the molar mass of helium is 4.0026x10-3 kg/mol. Find the absolute pressure of the helium gas. Pa Q A 2 N I W S #3 X H ption command E D 69 + $ 4 C R F G Search or type URL % 5 V T G MacBook Pro . .... I command option { [ ? 1 = "1 F O } 1arrow_forwardWhat volume is occupied by 200 g of oxygen under a pressure of 2.00 atm and a temperature of 310 K ? Πνα ΑΣφ Request Answer Submitarrow_forward
- A bar of aluminum is 1m long at a temperature of 400K. At what temperature in degrees K is the bar 0.999m in length? Use a an expansion coefficient of 25x10/degC for aluminum. 208K 312K 360K 403K esc 7. 00 2$ 4 %23 2 E R F G S C 36arrow_forward2 4. Calculate the number of gas molecules in a container of volume 0.30 m³ filled with gas under a partial vacuum of pressure 25 Pa at 17 °C. A I on N 2 W S #3 3. X H H command C E D $ 4 C R F % 5 G Search or type URL V T G A 832 11332 6 MacBook Pro B Y H & 7 N U J * 00 8 M 1 ( · 9 K O V H I ) O L command P > I : option K { [ ? Iarrow_forwardThe volume of an automobile tire is 2.5 × 10−2m3 . the pressure of the air in the tire is 3 atm and the temperature is 37C ◦ . what is is the mass of air in grams ? The mean molecular mass of air is 29g. 1 atm = 1.01 × 105 Pa ; Calculate to 2 decimals.arrow_forward
- 2. A vertical Cylinder with a heavy piston contains air at 300 K. The initial pressure is 3.35*10^5 Pa, and the initial volume is 0.300 m^3. Take the molar mass air as 28.9 g/mol and assume Cv = 5R/2, R = 8.314 J/mol K a) Find the specific heat of air at constant volume in units of J/kg C b) Calculate the mass of the air in the cylinder c)Suppose the piston is held fixed. Find the energy input required to raise the temperature of the air to 691K.arrow_forwardThe initial temperature of three moles of oxygen gas is 33.5°C, and its pressure is 7.80 atm. (a) What will its final temperature be when heated at constant volume so the pressure is two times its initial value? °C (b) Now the volume of the gas is also allowed to change. Determine the final temperature if the gas is heated until the pressure and the volume are doubled. °Carrow_forwardan aluminum aerosol can will burst if the pressure inside reaches 210.0 psi. If the can initially contains an ideal gas at 459.5 kPa at 298.15 K, at what temperature (in K) will the can burst?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON