
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Hydrogenation of
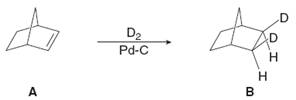
Transcribed Image Text:.D
D2
Pd-C
н
н
B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Synthesize (Z)-hept-5-en-2-one from ethyl acetoacetate (CH3COCH2CO2Et) and the given starting material. You may also use any other organic compounds or required inorganic reagents.arrow_forward(a) Draw the mechanism for the formation of both of the enols that can be formed from A (use acetic acid & AcOH as the source of the protons) (b) Draw the mechanism of reaction of this enol with bromine to give product Barrow_forwardRibose, a carbohydrate with the formula shown, forms a cyclic hemiacetal, which, in principle, could contain either a four-membered, five-membered, or six-membered ring. When D-ribose is treated with methanol in the presence of an acid catalyst, two cyclic acetals, A and B, are formed, both with molecular formula C,H,0, These are separated, and each is treated with sodium periodate (Section 10.8C) followed by dilute aqueous acid. Both A and B yield the same three products in the same ratios. он о CHO СНО H+ CH,OH A +B ÕH 1. NalO, 2. H,0* НО CHO + CHOH + CH,OH ÕH CH,OH Isomeric cyclic acetals with molecular formula CH12O, D-Ribose (C;H1605) From this information, deduce whether the cyclic hemiacetal formed by D-ribose is four- membered, five-membered, or six-membered.arrow_forward
- 1. (a) Propose structure of the major product when A is reacted with dil. H2SO4. (b) Propose a mechanism for the reaction. Show all intermediate structures and all electron flow with arrows. OH dil. H2SO4arrow_forward1,2-Diols are converted to carbonyl compounds when treated with strong acids, in a reaction called the pinacol rearrangement. (a) Draw a stepwise mechanism for this reaction. (Hint: The reaction proceeds by way of carbocation intermediates.) (b) Assuming that the pinacol rearrangement occurs via the more stable carbocation, draw the rearrangement product formed from diol D.arrow_forwardDevise a synthesis of CH3CH2C≡CCH2CH2CH3using CH3CH2CH=CH2as the starting material. You may use any other organic compounds or inorganic reagents.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY