
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Refer to image, please answer clearly and explain. Will rate!
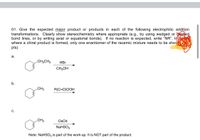
Transcribed Image Text:01. Give the expected major product or products in each of the following electrophilic addition
transformations. Clearly show stereochemistry where appropriate (e.g., by using wedged or shed
bond lines, or by writing axial or equatorial bonds). If no reaction is expected, write "NR". In
where a chiral product is formed, only one enantiomer of the racemic mixture needs to be show
pts)
а.
.CH2CH3
HBr
CH;OH
b.
.CH3
R(C=O)OOH
с.
CH3
Os04
NaHSO3
Note: NaHSO3 is part of the work-up. It is NOT part of the product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What does the following mechanism look like?arrow_forwardFor the following proton-transfer reaction, identify the correct curved arrow mechanism. 04.-04. H₂N 1 || ||| IV V -N-H + H H H H H H H H + H₂N + + + + H₂N^ H₂N^ H₂N H H₂N H + H3N1arrow_forwardplz dont round and take your time. this one has been getting done wrongarrow_forward
- You need to isolate a basic organic compound from a mixture of nonbasic compounds. This can be achieved by adding [Select] molecule to partition into the [Select] which will allow the resultingarrow_forwardTwo curved arrows are required to complete the given mechanism step. Refer to the numbered atoms (1, 2 and 4) and the numbered bond (3), as needed, to describe the positions where each arrow starts (tail) and ends (head). Select all that apply. 0 0 0 0 0 0 0 0 N:O: 3 One curved arrow starts at 4 and ends at 2. One One One curved arrow starts at 2 and ends at 4. curved arrow starts at 4 and ends at 1. curved arrow starts at 2 and ends at 1. One curved arrow starts at 3 and ends at 2. One curved arrow starts at 1 and ends at 2. One curved arrow starts at 1 and ends at 4. One curved arrow starts at 3 and ends at 4.arrow_forwardNonearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY