
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
![For the aqueous
[Cd(CN) 4] complex K₁ = 6.03 × 1018 at 25 °C.
Kf
Suppose equal volumes of 0.0098 M Cd (NO3)2 solution and 0.38M NaCN solution are mixed. Calculate the equilibrium molarity of aqueous
Round your answer to 2 significant digits.
M
x10
Cd2+
ion.](https://content.bartleby.com/qna-images/question/7bc30bfd-54a4-45be-8be6-a7bac17a47af/cbe7d3a6-7ce7-41ed-9e20-972e3076573f/bcyphtk_thumbnail.png)
Transcribed Image Text:For the aqueous
[Cd(CN) 4] complex K₁ = 6.03 × 1018 at 25 °C.
Kf
Suppose equal volumes of 0.0098 M Cd (NO3)2 solution and 0.38M NaCN solution are mixed. Calculate the equilibrium molarity of aqueous
Round your answer to 2 significant digits.
M
x10
Cd2+
ion.
Transcribed Image Text:.2+
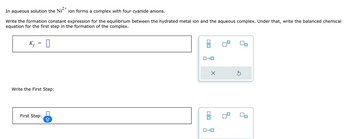
In aqueous solution the Ni ion forms a complex with four cyanide anions.
Write the formation constant expression for the equilibrium between the hydrated metal ion and the aqueous complex. Under that, write the balanced chemical
equation for the first step in the formation of the complex.
K₁ = ☐
Write the First Step:
First Step:
ロ→ロ
☑
ك
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 35 For the aqueous [Fe(CN), complex K₁=1.00 × 10³5 2+ Suppose equal volumes of 0.0078 M Fe(NO3), solution and 0.76M NaCN solution are mixed. Calculate the equilibrium molarity of aqueous Fe²+ ion. Round your answer to 2 significant digits. []м 2 x10 Śarrow_forward1. What is the pH of a 0.225 M solution of CH3NH2 (aq), for which Kb = 4.4 X 10 . 2. A buffer is prepared from 550 mL 0.250 M CH3COOH and 1.25 g NaOH (s). What is the pH of the buffer? Assume the volume does not change when the solid is added.arrow_forwardWrite the balanced chemical equation associated with the formation constant, Kf , for each complex ion. Include phase symbols. Ag(CN)2−: −⇀↽−↽−−⇀ BeF_(4)^(2)−: −⇀↽−↽−−⇀arrow_forward
- Help with the following questionarrow_forward[PBCI,] complex K; = 2.5 x 1015 For the aqueous at 25 °C. Suppose equal volumes of 0.0030M Pb(NO,) solution and 0.38M KCl solution are mixed. Calculate the equilibrium molarity of aqueous Pb- 2+ ion. Round your answer to 2 significant digits. ?arrow_forwardWrite the balanced chemical equation associated with the formation constant, Kf , for each complex ion. Include phase symbols. AgI_-(2): −⇀↽−↽−−⇀ Hg(NH3)2+4: −⇀↽−↽−−⇀arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY