
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:↑
...
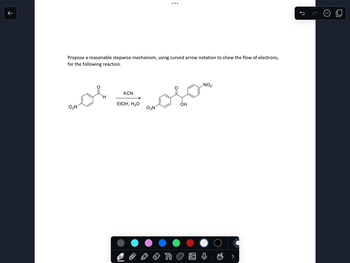
Propose a reasonable stepwise mechanism, using curved arrow notation to show the flow of electrons,
for the following reaction.
KCN
H
EtOH, H₂O
OH
02N
O₂N
NO2
Tt O ọ ♡ >
Ր
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 1 steps with 1 images

Knowledge Booster
Similar questions
- What is the product of the following Reaction?arrow_forwardName of product for each reactionarrow_forwardHO - CH, НО - ОН I CH_ - :0: solute CH ОН - NaCl ОН I CH - CH ОН - :0: || с :0: || - · C — CH2 — CH2 — C — ОН н Which is the better solvent? Н н C | H 1 C C 1 Н CH3- H2O CH₂OH C ||| C CH3(CH2), ОН :0: || S о- CH3 CH₂CH₂OH Н Нarrow_forward
- Choose the correct starting material for the following reaction. он MgBr MgBr H. A Barrow_forwardChoose the correct productarrow_forwardDraw the product for the following chemical reaction. Use the "Insert Image" button to add your hand-drawn work into the answer field." H H-C. -C- -H H HH + Cl₂arrow_forward
- What is the predicted major product of the reaction shown? hs O O O HO IV O || 1. NaOH/12 2. H3O+ HO OH V O |||arrow_forwardHO Which is the major product. NH2 NH2 NH2 HO H+ H ...OH H OH A B E: starting material N OH OH HOarrow_forwardRank the following alkenes in order of increasing rate of hydrogenation. 11 OI<|||< IV < II <|| < |||arrow_forward
- Complete the following reaction. H₂ A OH B FU O=O CH3 OH HO KMnO4 H₂O, heat C H₂ CH3 D OH CH H₂ CH3arrow_forward20) Propose reaction step(s) that would best accomplish the following conversions? OH O || || CH3CCH2CH H ? O || CH3CHCH2 CH OH CH3arrow_forwardPlease don't provide handwriting solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY