
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Can someone help me with these questions. Thank you
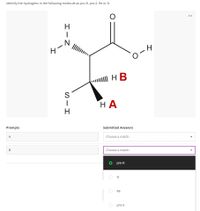
Transcribed Image Text:Identify the hydrogens in the following molecule as pro-R, pro-S, Re or Si
O-4
| HB
НА
Prompts
Submitted Answers
A
Choose a match
B
Choose a match
pro-R
Si
Re
pro-s
エーZ
の-エ
エ

Transcribed Image Text:Rank the following substituents from the highest to the lowest priority:
A. - CH3
B. - CH= CHCI
C. - CH2CI
D. -C= CCI
E. - CH2CH3
А) АЕBDC
B CDBEA
DBECA
D None of the above
E) АСЕBD
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 41 Select all functional groups present in the amino acid shown. (Hint: There will be more than one functional group present.) alcohol aldehyde alkene alkyne amine amide aromatic ring carboxylic acid ester ether ketone thiolarrow_forwardIf you were to use 6.00 g salicylic acid and an excess of acetic anhydride in the synthesis of aspirin, what would be the theoretical yield of acetylsalicylic acid in moles? In grams? Show your solution.arrow_forwardIs Tripamide soluable in waterarrow_forward
- The following Statin is called Pravastatin. It is used to help lower high levels of LDL-C in patients with Familial Hypercholesterolemia (FH). USE DIFFERENT COLORS FOR FOLLOWING QUESTIONS! Circle the Pharmacophore. Box the Functional groups.arrow_forwardWhat chemistry properties or structure make Salicylic acid cannot be taken orally? Is it due to the phenol functional group. Is hydroxyl (—OH) group attached to a carbon atom in a benzene ring what makes salicylic acid to be irritating? (please type clearly,please no blurry handwriting)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY