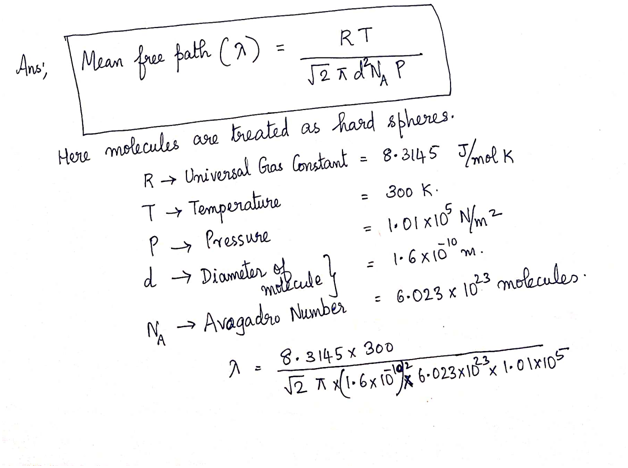
[11] An experimental balloon contains hydrogen gas (H2) at a temperature of 300K and a pressure of 1 atm (1.01 X 10° N / m?). (a) Calculate the mean-free path of a hydrogen molecule. Assume that a H2 molecule is effectively spherical, with a mean diameter of 1.6 X 1010 m. (b) Calculate the available volume per molecule (VI N), and find the average distance between each molecule and its nearest neighboring molecule (approximately the cube root of the available volume). Which is larger, the mean free path or the average nearest-neighbor distance between molecules? Exploring relationships
[11] An experimental balloon contains hydrogen gas (H2) at a temperature of 300K and a pressure of 1 atm (1.01 X 10° N / m?). (a) Calculate the mean-free path of a hydrogen molecule. Assume that a H2 molecule is effectively spherical, with a mean diameter of 1.6 X 1010 m. (b) Calculate the available volume per molecule (VI N), and find the average distance between each molecule and its nearest neighboring molecule (approximately the cube root of the available volume). Which is larger, the mean free path or the average nearest-neighbor distance between molecules? Exploring relationships
Related questions
Question
![[11] An experimental balloon contains hydrogen gas (H2) at a temperature of 300 K and a pressure of 1
atm (1.01 X 10° N / m?). (a) Calculate the mean-free path of a hydrogen molecule. Assume that a H2
molecule is effectively spherical, with a mean diameter of 1.6 X 1010 m. (b) Calculate the available
volume per molecule (VI N), and find the average distance between each molecule and its nearest
neighboring molecule (approximately the cube root of the available volume). Which is larger, the mean
free path or the average nearest-neighbor distance between molecules? Exploring relationships](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F9de2b0ed-01a2-4a8a-9b30-8cd619de91d2%2Fa85584e2-aee8-489a-ac76-d41118773cd2%2F5hr53l8_processed.png&w=3840&q=75)
Transcribed Image Text:[11] An experimental balloon contains hydrogen gas (H2) at a temperature of 300 K and a pressure of 1
atm (1.01 X 10° N / m?). (a) Calculate the mean-free path of a hydrogen molecule. Assume that a H2
molecule is effectively spherical, with a mean diameter of 1.6 X 1010 m. (b) Calculate the available
volume per molecule (VI N), and find the average distance between each molecule and its nearest
neighboring molecule (approximately the cube root of the available volume). Which is larger, the mean
free path or the average nearest-neighbor distance between molecules? Exploring relationships
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images
