
Concept explainers
(E) What does the number (+Z) at the center of each atom in Figure 1.1 represent, and whatnumber would you expect at the center of a representation of a bromine atom (Br)?
Interpretation:
The purpose of the representation of number (+Z) at the centre of each atom in given figure 1.1 should be determined along with the number which is present at the centre of a representation of bromine atom.
Concept Introduction:
In a planetary model of an atom, negative charged electrons are arranged around the positive charged electron in a series of shells which is like orbits.
The electrons present in the outermost energy level or shell is known as valence electrons. These electrons are available for bonding and outermost shell is known as valence shell.
Answer to Problem 1CTQ
+Z represents the nuclear charge of an atom which is equal to the number of protons or atomic number.
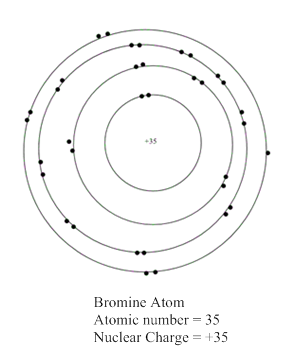
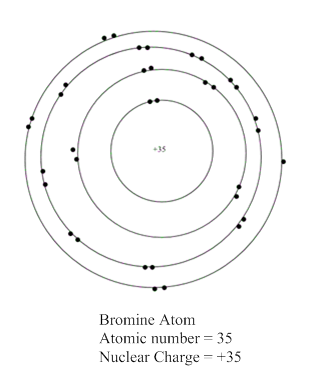
The representation of bromine atom:
Explanation of Solution
Given information:
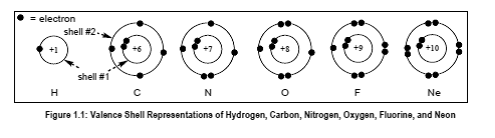
In the given figure, the number Z represents the atomic number of an atom such as atomic number of carbon is 6. +Z represents the nuclear charge which is nothing but atomic number.
The total charge present on all the protons in the nucleus is known as nuclear charge. The value of nuclear charge is equal to atomic number.
The number of protons present in nucleus of an atom is known as atomic number.
Now, atomic number of bromine atom is equal to 35 which is equal to the number of protons present in bromine atom. In the valence shell representation, +35 is present at the centre of a representation of bromine atom.
Hence, the representation of bromine atom is shown as:
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry: A Guided Inquiry
Additional Science Textbook Solutions
Chemistry: A Molecular Approach (4th Edition)
General Chemistry: Principles and Modern Applications (11th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Chemistry In Context
Chemistry: The Central Science (14th Edition)
- What neutral atom is represented by the electron configuration in Figure 3.2?arrow_forwardQuestion 14 Simplifying each Cl as a spherical donor (instead of considering all the s and p orbitals), derive a reducible representation for the two Cl atoms in CH₂Cl₂. (Enter your answer as a string of numbers with no spaces or additional characters and make sure to use the Cartesian coordinate system provided above.) Question 15 Decompose the reducible representation in Q14 (for the two Cl atoms) into a sum of irreducible representations. (Enter your answer in the format {capitalized Mullikan symbol}{space}{+}{space}{capitalized Mullikan symbol} etc. For example: A1g + A2g)arrow_forwardState whether it is R or S configuration and show how you found it.arrow_forward
- Chemistry This is a molecule of aspirin, a very commonly used painkiller. Draw up a table to show the KEY peaks that you would expect to see in the infrared spectrum of aspirin, identifying which bond has given rise to which peak.arrow_forward(ii) What are the two parts of a wavefunction? (iii) What is the importance of squaring a wavefunction? (iv) Where is a wavefunction obtained from? QUESTION 4 Draw the best Lewis structures for the substances below. Show your thinking and method. (a) (b) Oxalic acid, H2C2O4 Bromate ion, BrO3- XEOF2 CH3CN QUESTION 5 Methanol (CH3OH) is a liquid at room temperature with a density of 7.91 ×102 kg/m³. In a certain experiment, from the reaction of 2.91 mL of methanol with 2.88 g of oxygen, 2.27 g of carbon dioxide was obtained. (a) (b) What type(s) of chemical reaction is (are) occurring in this experiment? What equipment do you think was used to measure the volume of methanol in this experiment? Calculate the percent yield of the carbon dioxide in this experiment. (c) 5arrow_forwardDetermine the number of different kinds of protons in each compound.(a) 1-chloropropanearrow_forward
- 1. Which proton has the largest multiplicity? Smallest multiplicity?arrow_forwardlonization Energy Identify the neutral species, the species that would have a -3 charge with this spectrum and a +3 charge with this spectrum Identify the neutral species, the species that would have a -3 charge with this spectrum and a +3 charge with this spectrum O Kr°, As3+, Y3- O Kr, As-, Y3+ O Arº, P3-, sc+ OXeº, Sb , La+ Number of Electronsarrow_forwardDraw a model, like those in Figure , for CH4. Hint: Carbon has two electrons in its inner shell and four in its second shell.arrow_forward
- 5) Given the absorbance spectrum shown below, what wavelength of light would you use for nickel(II) ion? Explain your answer. Absorbance 0.8 0.6 0.4 1 0.2 0 350 400 Absorbance vs. Wavelength for Nickel(II) ion 450 500 550 600 650 Wavelength (nm) 700 750 800 850arrow_forwardNeed help with H, getting confused how many groups there is. I got 6 groupsarrow_forward5. A sample of a red dye and a sample of a blue dye both have the same concentration. Their Absorbance is measured using the same spectrophotometer. At a particular wavelength, will both samples give the same Absorbance reading? Why or why not?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
