When 2,2-dimethylcyclohexanol is treated with acid, 1,2-dimethylcyclohexene and iso- propylidenecyclopentane are the products obtained. Draw a detailed mechanism that explains this result. CH3 H3O+ CH3 CH3 CH3 OH CH3 CH3 2,2-dimethyl- cyclohexanol 1,2-dimethyl- isopropylidene- cyclopentane cyclohexene
When 2,2-dimethylcyclohexanol is treated with acid, 1,2-dimethylcyclohexene and iso- propylidenecyclopentane are the products obtained. Draw a detailed mechanism that explains this result. CH3 H3O+ CH3 CH3 CH3 OH CH3 CH3 2,2-dimethyl- cyclohexanol 1,2-dimethyl- isopropylidene- cyclopentane cyclohexene
Chapter22: Carbonyl Alpha-substitution Reactions
Section22.SE: Something Extra
Problem 27MP
Related questions
Question
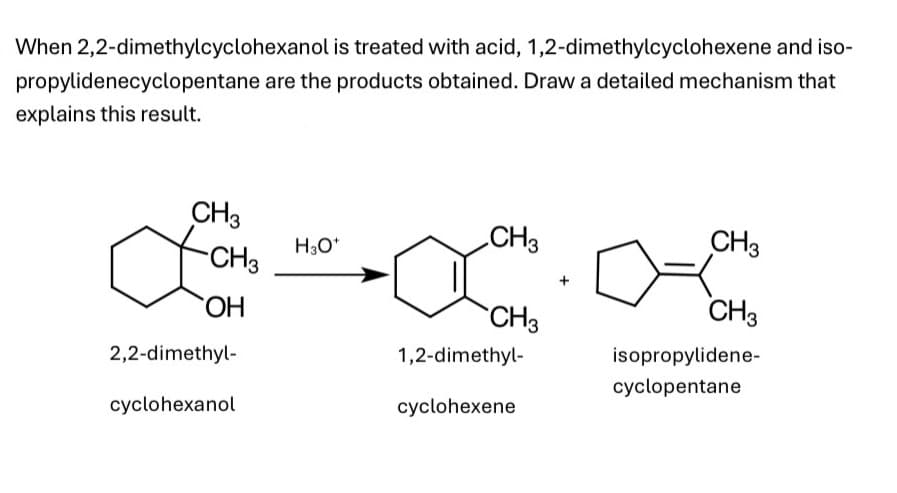
Transcribed Image Text:When 2,2-dimethylcyclohexanol is treated with acid, 1,2-dimethylcyclohexene and iso-
propylidenecyclopentane are the products obtained. Draw a detailed mechanism that
explains this result.
CH3
H3O+
CH3
CH3
CH3
OH
CH3
CH3
2,2-dimethyl-
cyclohexanol
1,2-dimethyl-
isopropylidene-
cyclopentane
cyclohexene
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning