The Molarity of Acetic Acid in Vinegar Enter the values not given. Use units as appropriate, and enter using regular notation with the correct sf. Use leading zeroes if a number is less than 1. Data used = Trial _1__ Trial _2__ -- 2.6334 mol 2.4244 mol Moles of base used in titration Moles of acetic acid neutralized 2.6334 mol 2.4244 mol in vinegar sample Molarity of acetic acid in vinegar 0.5268 M 0.4864 M 0.5066 M Average Molarity
The Molarity of Acetic Acid in Vinegar Enter the values not given. Use units as appropriate, and enter using regular notation with the correct sf. Use leading zeroes if a number is less than 1. Data used = Trial _1__ Trial _2__ -- 2.6334 mol 2.4244 mol Moles of base used in titration Moles of acetic acid neutralized 2.6334 mol 2.4244 mol in vinegar sample Molarity of acetic acid in vinegar 0.5268 M 0.4864 M 0.5066 M Average Molarity
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 102AP
Related questions
Question
|
Trial 1 |
Trial 2 |
||
|---|---|---|---|
|
Initial Buret Reading |
0.05 mL | 0.02 mL | |
|
Final Buret Reading |
25.25 mL | 23.22 mL | |
|
Volume of base used |
|||
|
Molarity of base used |
0.1045 M | 0.1045 M | |
|
Volume of vinegar used |
5.00 mL | 5.00 mL |
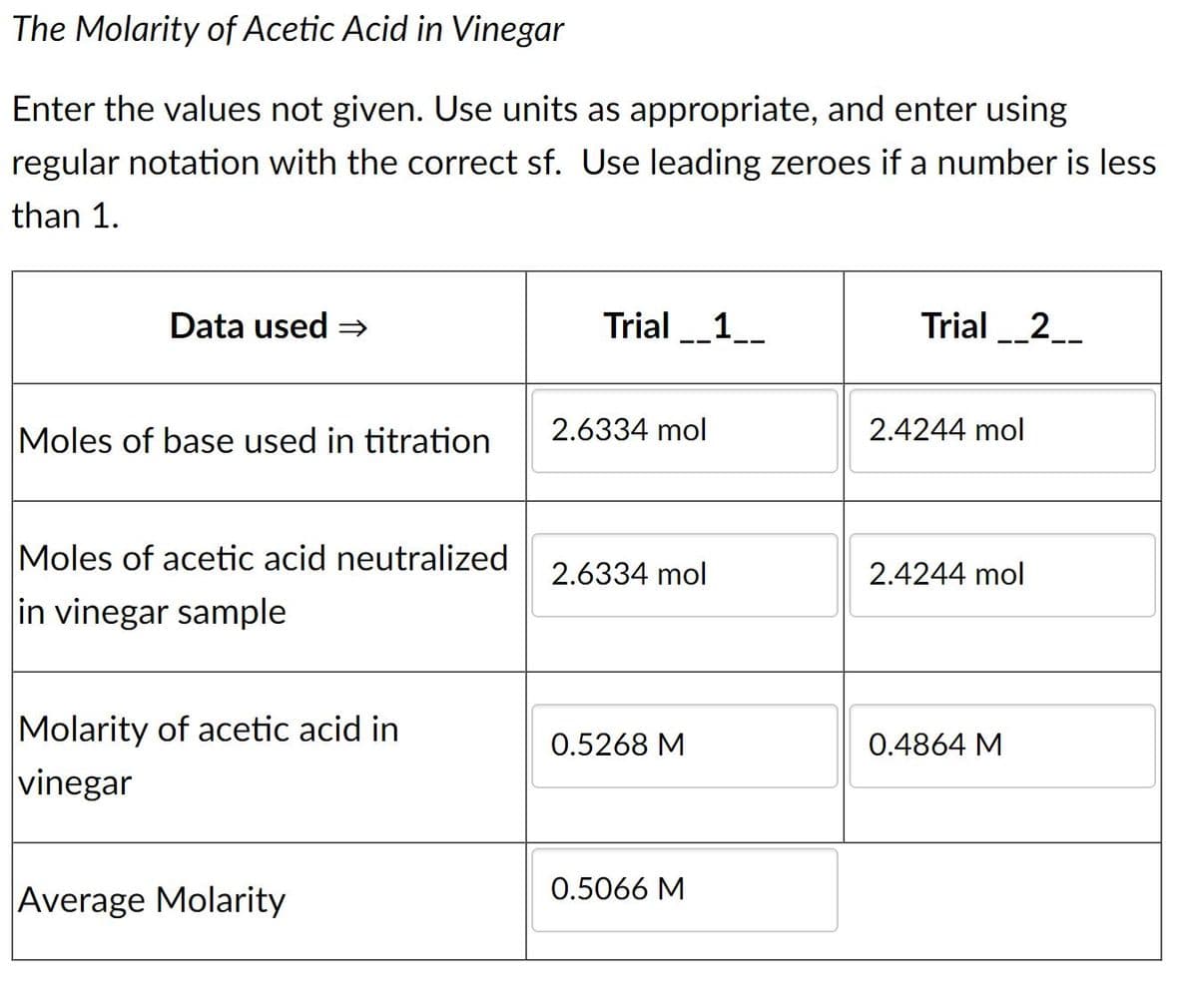
Transcribed Image Text:The Molarity of Acetic Acid in Vinegar
Enter the values not given. Use units as appropriate, and enter using
regular notation with the correct sf. Use leading zeroes if a number is less
than 1.
Data used =
Trial _1__
Trial _2__
2.6334 mol
2.4244 mol
Moles of base used in titration
Moles of acetic acid neutralized
2.6334 mol
2.4244 mol
in vinegar sample
Molarity of acetic acid in
0.5268 M
0.4864 M
vinegar
0.5066 M
Average Molarity
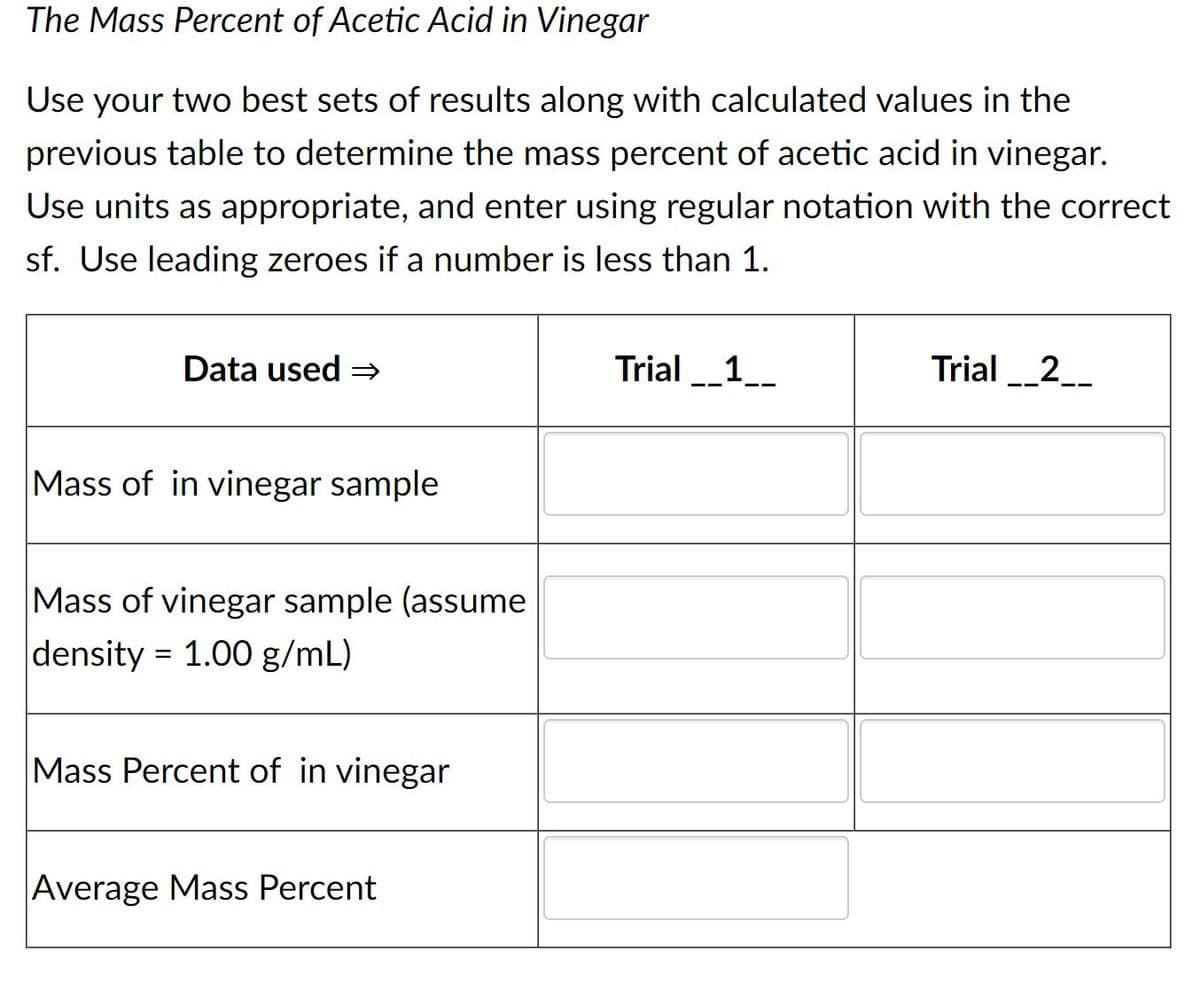
Transcribed Image Text:The Mass Percent of Acetic Acid in Vinegar
Use your two best sets of results along with calculated values in the
previous table to determine the mass percent of acetic acid in vinegar.
Use units as appropriate, and enter using regular notation with the correct
sf. Use leading zeroes if a number is less than 1.
Data used =
Trial_1_
Trial _2_
Mass of in vinegar sample
Mass of vinegar sample (assume
density = 1.00 g/mL)
Mass Percent of in vinegar
Average Mass Percent
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning
