Suppose a 500. mL flask is filled with 0.40 mol of CH4, 0.30 mol of H₂S and 0.10 mol of CS2. This reaction becomes possible: CH(g)+2H,S(g) CS2(g)+4H2(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CS2. You can leave out the M symbol for molarity. initial change equilibrium CS₂ H₂ CH₁ H,S છું" 0 n 0 D X 0 ㅁㅁ X 5
Suppose a 500. mL flask is filled with 0.40 mol of CH4, 0.30 mol of H₂S and 0.10 mol of CS2. This reaction becomes possible: CH(g)+2H,S(g) CS2(g)+4H2(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CS2. You can leave out the M symbol for molarity. initial change equilibrium CS₂ H₂ CH₁ H,S છું" 0 n 0 D X 0 ㅁㅁ X 5
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.77PAE: The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce...
Related questions
Question
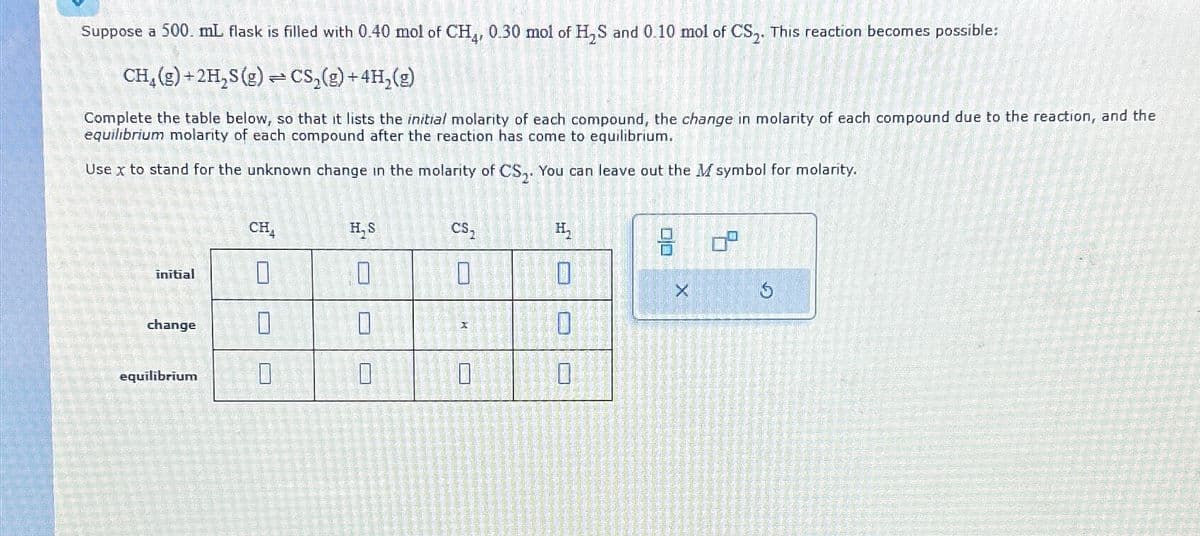
Transcribed Image Text:Suppose a 500. mL flask is filled with 0.40 mol of CH4, 0.30 mol of H₂S and 0.10 mol of CS2. This reaction becomes possible:
CH(g)+2H,S(g)
CS2(g)+4H2(g)
Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the
equilibrium molarity of each compound after the reaction has come to equilibrium.
Use x to stand for the unknown change in the molarity of CS2. You can leave out the M symbol for molarity.
initial
change
equilibrium
CS₂
H₂
CH₁
H,S
છું"
0
n
0
D
X
0
ㅁㅁ
X
5
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning