Substance Specific Heat Capacity (J/g °C) 1. ethylene glycol 2.47 2. brass 0.377 3. glass 0.837 4. vegetable oil 1.97 A 50 g sample of each of the above materials, each with an initial temperature of 21.0°C, had the same quantity of thermal energy added to them. If they were listed from the highest final temperature reached to the lowest final temperature reached, the order would be O and 1 23 660 41 of thermal energy are added to 200 4 vegetable oil in a fondue pot. The vegetable oil had an initial
Substance Specific Heat Capacity (J/g °C) 1. ethylene glycol 2.47 2. brass 0.377 3. glass 0.837 4. vegetable oil 1.97 A 50 g sample of each of the above materials, each with an initial temperature of 21.0°C, had the same quantity of thermal energy added to them. If they were listed from the highest final temperature reached to the lowest final temperature reached, the order would be O and 1 23 660 41 of thermal energy are added to 200 4 vegetable oil in a fondue pot. The vegetable oil had an initial
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 14P
Related questions
Question
Hello! I have attached an question and can you please please help answer it for me? Thank you so much :)
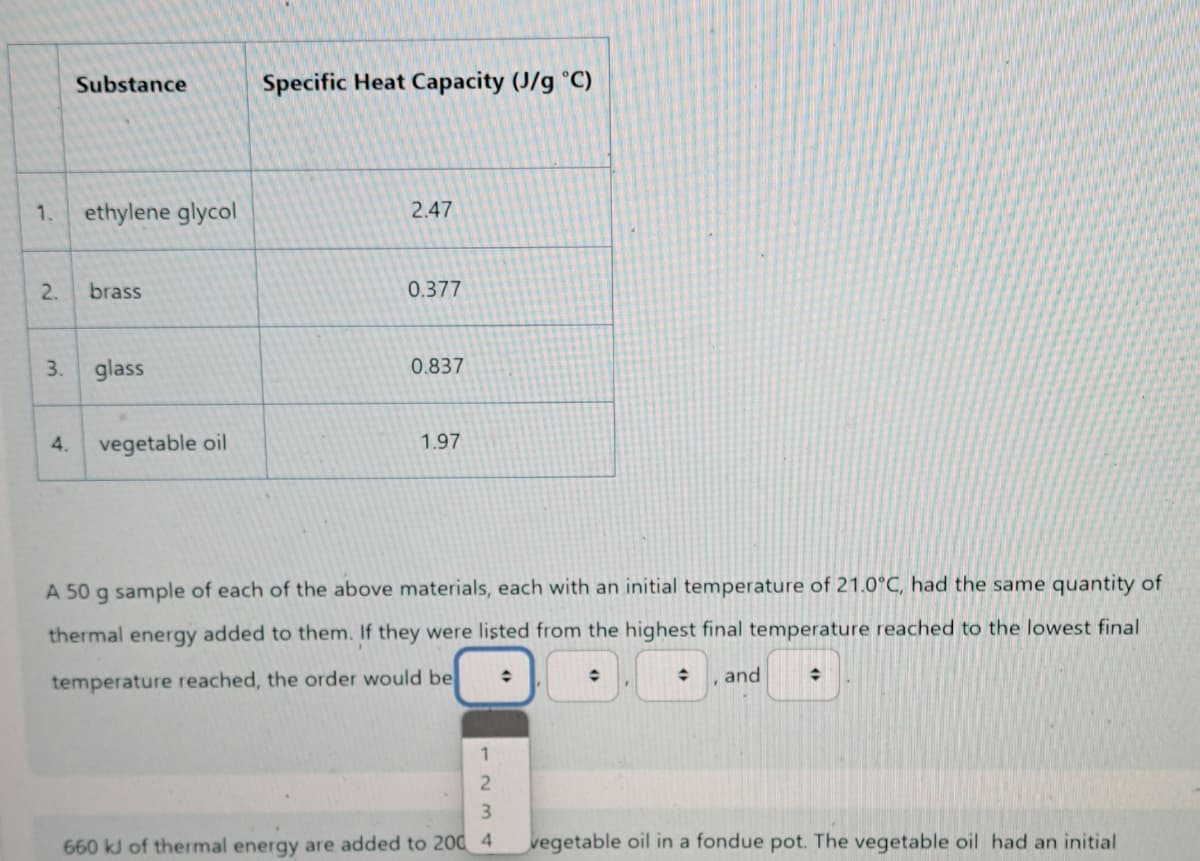
Transcribed Image Text:Substance
Specific Heat Capacity (J/g °C)
1.
ethylene glycol
2.47
2.
brass
0.377
3.
glass
0.837
4.
vegetable oil
1.97
A 50 g sample of each of the above materials, each with an initial temperature of 21.0°C, had the same quantity of
thermal energy added to them. If they were listed from the highest final temperature reached to the lowest final
temperature reached, the order would be
and
1234
660 kJ of thermal energy are added to 200 4 vegetable oil in a fondue pot. The vegetable oil had an initial
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning