Fill in the major products to complete the transformations. Be sure to pay careful attention to stereochemistry where appropriate. If the major product is a pair of enantiomers or diastereomers be sure to draw them both. And indicate whether you have drawn an enantiomer or a diastereomer. (Don't forget about rearrangement).
Fill in the major products to complete the transformations. Be sure to pay careful attention to stereochemistry where appropriate. If the major product is a pair of enantiomers or diastereomers be sure to draw them both. And indicate whether you have drawn an enantiomer or a diastereomer. (Don't forget about rearrangement).
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter10: Acids, Bases, And Salts
Section: Chapter Questions
Problem 10.81EP
Related questions
Question
Fill in the major products to complete the transformations. Be sure to pay careful
attention to stereochemistry where appropriate. If the major product is a pair of enantiomers or diastereomers be sure to draw them both. And indicate whether you have drawn an enantiomer or a diastereomer. (Don't forget about rearrangement).
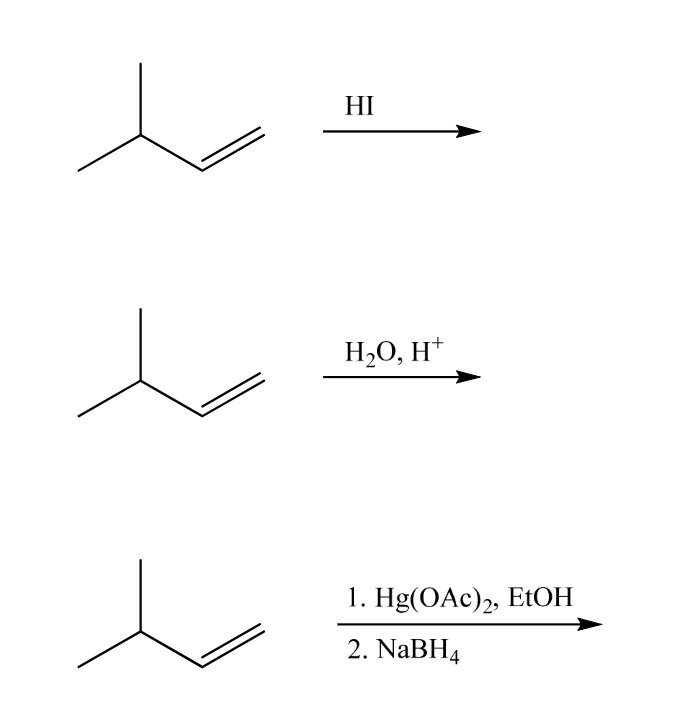
Transcribed Image Text:HI
H₂O, H+
1. Hg(OAc)2, EtOH
2. NaBH4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
shouldn't a hydride shift take place for the HI and the tH20, H+ reactions in order to get the major product. Also please draw the enantiomers and diastereomers products (if there are any) and if there aren't explain why for all the reactions
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning