Q: ture content determination? What are the USP and NF official methods of determining moisture content...
A: As per rule, allowed to answer first question and post the remaining in the next submission. The q...
Q: There are two naturally occurring isotopes of chlorine. 35 Cl has a mass of 34.9689 u. 37 Cl has a m...
A: We have to find the percentage abundance of 35Cl and 37Cl.
Q: he following reaction and its equilibrium constant: I2(8) = 2 1(g) Kp = 0.209 %3D A reaction mixture...
A:
Q: Gaseous methane (CH,) reacts with gaseous oxygen gas (o, to produce gaseous carbon dioxide (CO,) and...
A:
Q: In the given three-dimensional molecular structure, the IC OH N differently colored spheres represen...
A:
Q: A certain solution consists of isopropyl alcohol diluted in water. If a 500 mL bottle contains 75 mL...
A: The volume of solute in milliliter present in 100 mL solution is known as the volume percent concent...
Q: 5. Show how you would synthesize the following compound beginning with the given starting material. ...
A:
Q: Which of the following pairs of compounds will react to form a precipitate? Why? calcium chloride a...
A: The solubility of an ionic compound in water depends on the nature of the ions present in the ionic ...
Q: Consider the following chemical equilibrium: 2 PbO (s) +O2 (g) 2 PbO, (s) Now write an equation belo...
A:
Q: Which of the compounds below will form hydrogen bonds between two of the same molecules? CH3NHC...
A: Hydrogen bonding:The hydrogen atom attached with the electronegative atom like fluorine, oxygen, or ...
Q: A polluted stream flows at 1 MGD carrying a concentration of 1 mg/L of a conservative pollutant such...
A: Given that, a polluted stream flows at 1 MGD (million gallons per day) carrying a concentration of 1...
Q: 7. In each set of structures, arramge_the labeled bonds in the order indicaled Set 111 CH2 CHe 91 H-...
A: A question based on molecules that is to be accomplished.
Q: Which of the following are true? Chose all the correct answers. O 1,3-Cis disubstituted cyclohexane ...
A: When the groups are present on the equotorial position are more stable than axial position. In axial...
Q: catalyst speeds up a chemical reaction by lowering the reaction’s activation energy. Explain why/how...
A: The activation energy and the rate of reaction are inversly proportional. And catalalyst plays a maj...
Q: Use the observations about each chemical reaction in the table below to decide the sign (positive or...
A: A question based on chemical thermodynamics that is to be accomplished.
Q: Suppose the galvanic cell sketched below is powered by the following reaction: Ni(s)+Pb(NO,),(aq) Ni...
A:
Q: MULTIPLE CHOICE QUESTION Thomson's idea of the atom looked like chocolate chip cookies because elect...
A: JJ Thomson give atomic model and describe that two types of particles ( proton and neutron ) is pres...
Q: 5. Calculate both [H'] and [OH] for the following solutions at 25°C. Be sure to clearly identify all...
A:
Q: What is the order of a reaction with respect to a reactant "A" if a plot of ln[A] vs time is linear?...
A:
Q: Attempt 3 Some of the formulas could be either molecular or empirical formulas; however, some can on...
A: A molecular formula can be defined as the chemical formula that represents the total number of atoms...
Q: Classify each element. Note that another term for main group is representative, another term for sem...
A:
Q: Consider the same reaction carried out two more times, both at the same condition(including concentr...
A:
Q: partially hydrogenated vegetable oil
A: In this question we have to explain the equation for baeyers , bromine and sulfuric acid, and flamma...
Q: 2. A 0.4682 g sample of a metal is dissolved in a total volume of 50.0 mL. a. If the metal was pure ...
A: a) The Ag and KSCN reacts in following way: Ag+KSCN→Ag(SCN) + K Mass of Ag =0.4682 g Moles of Ag is...
Q: Which one of the equations below represents what happens when HC,H3O2 is dissolved in water? O HC2H3...
A:
Q: Proposed a mechanism on how to synthesize 2, 3 dimethylbutane
A: Given that Synthesis of 2, 3 dimethylbutane from propane
Q: The rate of a certain reaction is given by the following rate law: rate=k[N,][H,] Use this informati...
A: Chemical kinetics can be defined as the branch of chemistry that deals with rates of chemical reacti...
Q: A reaction, 2A + 3B ------> 2 C, obeys the rate law: r = k[A][B]2. What is the order of this react...
A: Given :- 2A + 3B ------> 2 C rate law: r = k[A][B]2 To determine :- overall reaction order
Q: In an experiment to determine the rate law, the rate of the reaction was determined to be 0.476 Ms¯1...
A:
Q: Locke-Ringer's solution contains 9 g of sodium chloride, 0.24 g of calcium chloride, 0.42 g of potas...
A: Locke-Ringer Solution An isotonic solution used for the treatment of dehydration. It contains diffe...
Q: Given the equation below, which of the following is true? 2 Mg(s) + O2(g) → 2 MgO(s) + 72.3 kJ 12. A...
A: 2Mg + O2->2MgO+72.3KJ
Q: A reverse osmosis desalination plant treats 20 MGD (million gallons per day) brackish water. The wat...
A:
Q: What is the freezing point depression of a 1.50 m solution of sucrose in water? No need to include t...
A: Given :- molality of sucrose solution = 1.50 m To be calculated :- freezing point depression
Q: 8. Select the endothermic reaction. CH4(s) + O2(g) → CO>(g)+ 2 H,O(1) + 890 kJ 2 HC1(g) + 185 kJ → H...
A:
Q: QUESTION 3 Which of the following conjugate bases are correct? There may be more than one correct an...
A:
Q: 5- In each part, choose the structure that best adapts to the infrared spectrum
A: g) In this case, the structure should be Reason: simply base value for isolated aldehyde peaks in I...
Q: Is the molecule below polar or non-polar? Why? Hint: the electronegativity of Ec is 3.4 :F-Ec =N: :F...
A: Concept; Dipole moment equal to zero called the non-polar molecule . Dipole moment not equal to zer...
Q: he H NMR spectrum of a molecule with molecular formula C3H,Cl, has a quintet at 2.19 ppm, and a trip...
A:
Q: Are all atoms of an element exactly the same? Maybe No HUH? Yes
A: We need to determine if the atoms of same element are same.
Q: Match the evidence and reasoning to support the claim: Light is a particle. Evidence: I. Light passi...
A:
Q: Heptane: yellow flame
A: This is a multipart question.According to the guidelines, in multiple parts question, we can solve o...
Q: Write the combustion reaction for C2H6
A:
Q: Which of the compounds below will form hydrogen bonds between two of the same molecules? O CH3CH2CH2...
A: Please note- As per our company guidelines we are supposed to answer only one question. Kindly repos...
Q: Suppose a 0.48M aqueous solution of phosphoric acid (H,PO,) is prepared. Calculate the equilibrium m...
A: Given, Concentration of aqueous Phosphoric acid solution = 0.48 M Required, ...
Q: concentration of the solution. (c) the mass in grams of NiCl2 contained in each liter O. A 12.5% (w/...
A: As per our guideline, only one to be solved, if multiple questions are posted. Hence, question no. 9...
Q: 4. The variance of many measurements of observable A is given by o? = (A²) – (A)². Derive the varian...
A: We have to derive the measurement in the variation of the position of a particle in a box in a state...
Q: Consider the colors found in the orange pen. Choose the Rf value closest to that of the spot that tr...
A: A question based on analytical separations that is to be accomplished.
Q: Suppose a 0.48M aqueous solution of phosphoric acid (H,PO,) is prepared. Calculate the equilibrium m...
A:
Q: Which of the following statements is correct about the Electrodes of the first kind Many of them dis...
A: A question based on electrochemical cells that is to be accomplished.
Q: Electrophilic addition of HI
A:
![Express the equilibrium constant for the following reaction.
7 H 2(8) + 7 Br 2( g) = 14 HBr( g)
[H2] [Br2]
K =
[HBr?
[HBr]14
K =
H2B2
[H2][Br2]
K =
[HBr]
[HBr]
K =
[H2 Br27
[HBr]14
K =
10:37 AM
2/20/2022](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F39a6c793-b207-4945-b9e4-d211323cb51c%2F05a2e5d6-4e31-44af-9306-fd8d57ac812f%2F9vf18pb_processed.jpeg&w=3840&q=75)
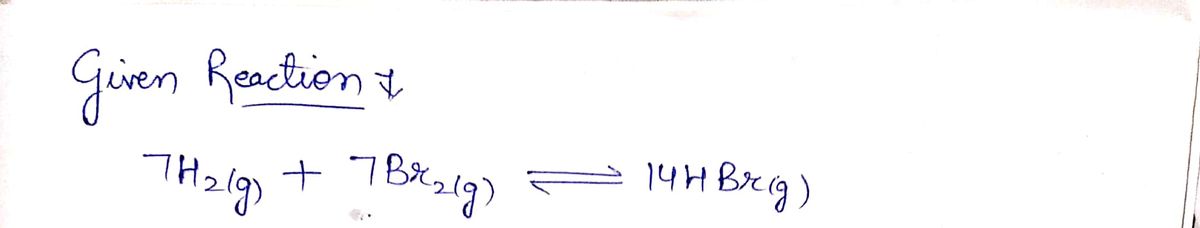
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

- the equilibrium expressions for the following reactions: 2Fe(s) + 3H2O(g) ↔ Fe2O3(s) + 3 H2(g) A [Fe2O3][H2] = Keq [Fe][H2O] B [Fe2O3][H2]3 = Keq [Fe]2 [H2O]3 C [H2]3 = Keq [H2O]3 D [Fe]2 [H2O]3 = Keq [Fe2O3][H2]3Express the equilibrium constant for the following reaction. CH4(g) + O2(g) = CO2(g) + 2 H2O(g) [CH4][02] A) K= [CO2][H2O] [CH4][202]² B) K= [CO2][2H2012 [CO2][H2012 C) K = [CH4][02]² [CO2][2H2012 D) K = [CH4][202]² [CH4][02]² E) K = [CO2][H2012 ○ A) B) D) ○ E)Write the expression for the equilibrium constant for the reaction before you can calculate for the Keq or Kc: 2 P₂O5(g) 4 PO₂(g) + O₂(g) Calculate the equilibrium constant if the equilibrium concentrations are: [P₂O5] = 0.63 mol/L. [PO₂] = 0.74 mol/L, [O₂]= 0.21 mol/L.
- Write the equilibrium constant expression for the following reaction:C6H12O6(s)+6O2(g)↔6CO2(g)+6H2O(g) a. [H2O]^6 / [C6H12O6][O2]^6 b. [CO2]^6[H2O]^6 / [C6H12O6] c. ([CO2]^ 6 / [C6H12O6][O2]^6 d. None of these. e. [CO2]^6[H2O]^6 / [O2]6What is the equilibrium expression for the reaction below? 4M(s) + Y2(g) +→ 2M2Y(s) Periodic Table and Datasheet 1 Kc [Y2] O Kc = [Yz] [M2Y]? Kc [M]¶[Y2] [M1*[Y2] Kc [M2Y]?Consider the following reaction: CH4(g) + 2 H2S(g) = CS2(g) + 4 H2(g) A reaction mixture initially contains 0.50 M CH4 and 0.75 M H2S. If the equilibrium concentration of H2 is [H2]eq 0.44 M, find the equilibrium constant (Kc) for the reaction.
- At equilibrium, the concentration of N2 is 5.32x10-3 M, O2 is 3.28 x10-3 M and that of NO is 6.19 x10-6 M. Evaluate the equilibrium constant for this reaction.Complete the two acid dissociation reactions for the ethylenediammonium ion and select the correct symbol for the equilibrium constant for each reaction. Step 1: NHẸCH,CH,NH(aq) — What is the symbol for the equilibrium contant for step 1? O Kb2 O Ka2 Ο Και Kbl Step 2: NH₂CH₂CH₂NH3(aq) = What is the symbol for the equilibrium constant for step 2?2. Which of the following statements about reaction quotient (Qc) is FALSE? a. The equation used is aA + bB is reversible by cC + dD B. It is used to determine if chemical equilibrium is C. If Qc < Kc, the products dominate in the chemical D. If chemical equilibrium is not achieved, Qc is used to determine where the direction of reaction will proceed in order to attain the 3. If the computed Kc of an unknown compound is equal to 3, where does chemical equilibrium lie? a. center b. left C. right D. left to right
- At 25 °C, the following reactions have the equilibrium constants noted to the right of their equations. 2CO(g) + O₂(g) ⇒ 2CO₂(g) Kç= 3.3 × 10⁹1 2H₂(g) + O₂(g) → 2H₂O(g) Kc= 9.1 × 108⁰ Use these data to calculate Kc for the reaction H₂O(g) + CO(g) ⇒ CO₂(g) + H₂(g) Kc= i11. Given the following equilibria and equibrium constants: 1/2 N₂ (g) + 1/2 O₂ (g) NO (g) Kc = 4.8 × 10-10 2 NO₂ (g) 2 NO(g) + O₂(g) Kc = 1.1 x 10-5 | a. What is the value of Ke for the following reaction? Show your work! 2 NO₂ (g) N₂ (g) + 2 O₂ (g) Kc = b. In a container, [NO₂] = [N₂] = [0₂] = 1.000 × 10-5 M. Prove or disprove that the mixture in the container is at equilibrium. If the mixture is not at equilibrium, explain which species will change concentration, and how those concentrations will change.9) Which of the following is the correct equilibrium law for the following reaction? N₂(g) + 3H₂(g) = 2NH3(g) [N₂] [H₂]3 [NH₂12 B) Kc = [NH3]2[N₂] [H₂]3 [NH3] [N₂] [H₂] A) Kc = C) Kc = [NH3 ]2 [N₂] [H₂]³ E) Ke= [NH3] [N₂] [H₂] D) Kc =





