Part B Calculate the magnitude of the current in amperes if the number of moles of hydrogen gas produced is 5.32×10-4 mol (from part A). Express your answer to three significant figures and include the appropriate units. ► View Available Hint(s) μA Value Units Submit Previous Answers www ? * Incorrect; Try Again; One attempt remaining
Part B Calculate the magnitude of the current in amperes if the number of moles of hydrogen gas produced is 5.32×10-4 mol (from part A). Express your answer to three significant figures and include the appropriate units. ► View Available Hint(s) μA Value Units Submit Previous Answers www ? * Incorrect; Try Again; One attempt remaining
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Our experts need more information to provide you with a solution. Please resubmit your question, making sure it's detailed and complete. We've credited a question to your account.
Your Question:
Please type answer no write by hend.
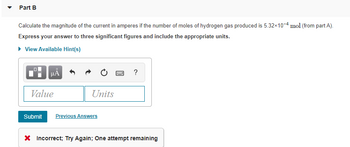
Transcribed Image Text:Part B
Calculate the magnitude of the current in amperes if the number of moles of hydrogen gas produced is 5.32×10-4 mol (from part A).
Express your answer to three significant figures and include the appropriate units.
► View Available Hint(s)
μA
Value
Units
Submit Previous Answers
www
?
* Incorrect; Try Again; One attempt remaining
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
