Part B A solution is made by mixing 12.5 g of Sr(OH)2 and 55.0 mL of 0.250 M HNO3. Calculate the concentration of OH ion remaining in solution. Express your answer using three significant figures. concentration OH remaining = Submit Request Answer Part C Ο ΑΣΦ 13 ? Calculate the concentration of Sr2+ ion remaining in solution. Express your answer using three significant figures. Ο ΑΣΦ concentration Sr2+ remaining = Submit Request Answer Part D Calculate the concentration of NO3 ion remaining in solution. Express your answer using three significant figures. oncentration NO3 remaining = Submit Request Answer Part E ΑΣΦ 0 ? M M M
Part B A solution is made by mixing 12.5 g of Sr(OH)2 and 55.0 mL of 0.250 M HNO3. Calculate the concentration of OH ion remaining in solution. Express your answer using three significant figures. concentration OH remaining = Submit Request Answer Part C Ο ΑΣΦ 13 ? Calculate the concentration of Sr2+ ion remaining in solution. Express your answer using three significant figures. Ο ΑΣΦ concentration Sr2+ remaining = Submit Request Answer Part D Calculate the concentration of NO3 ion remaining in solution. Express your answer using three significant figures. oncentration NO3 remaining = Submit Request Answer Part E ΑΣΦ 0 ? M M M
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 139SCQ: Two students titrate different samples of the same solution of HCl using 0.100 M NaOH solution and...
Related questions
Question
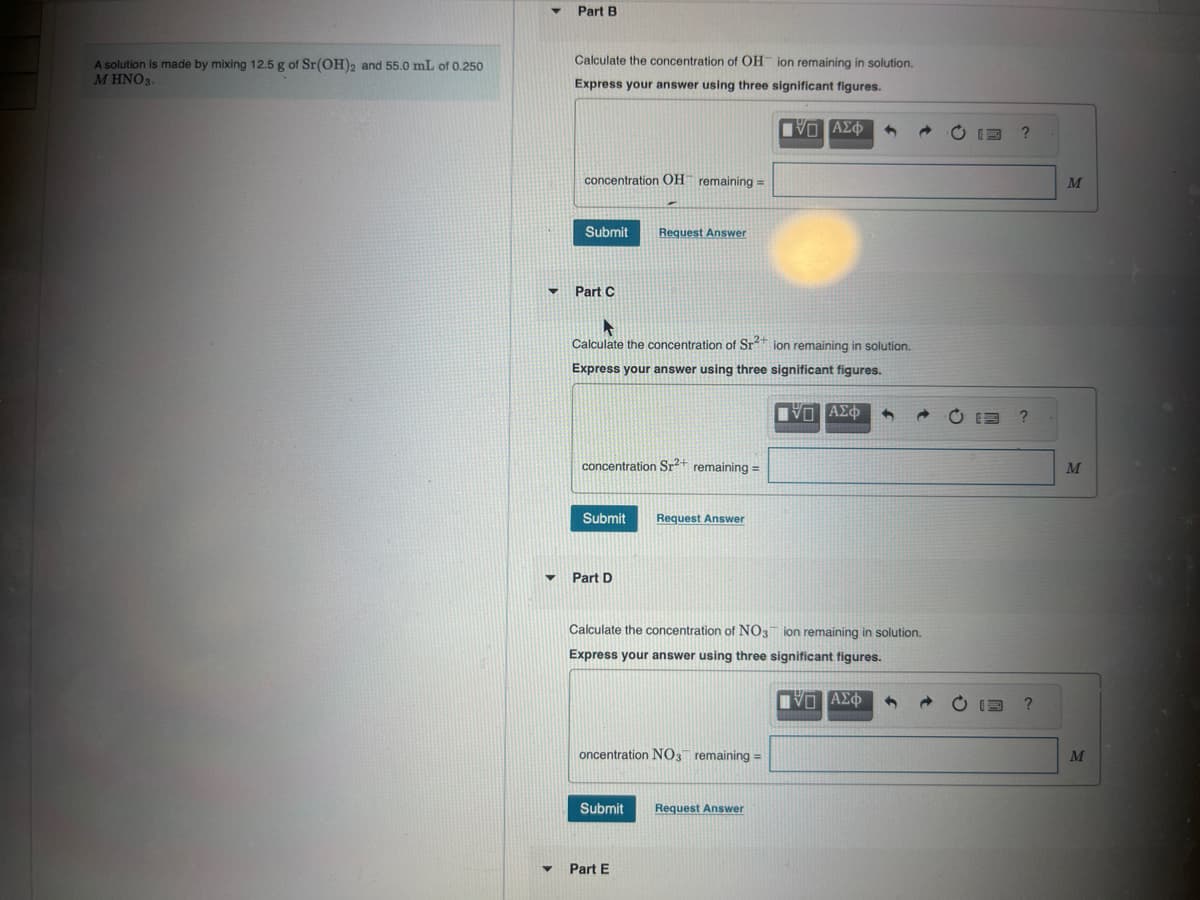
Transcribed Image Text:Part B
A solution is made by mixing 12.5 g of Sr(OH)2 and 55.0 mL of 0.250
M HNO3.
Calculate the concentration of OH ion remaining in solution.
Express your answer using three significant figures.
concentration OH remaining =
Submit Request Answer
Part C
Ο ΑΣΦ
13
?
Calculate the concentration of Sr2+ ion remaining in solution.
Express your answer using three significant figures.
Ο ΑΣΦ
concentration Sr2+ remaining =
Submit
Request Answer
Part D
Calculate the concentration of NO3 ion remaining in solution.
Express your answer using three significant figures.
oncentration NO3 remaining =
Submit
Request Answer
Part E
ΑΣΦ
0
?
M
M
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps with 4 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning