most of the additions of bromine to double bonds gave entirely antistereochemistry. Explain why the addition to phenanthrene gives a mixture of synand anti stereochemistry
most of the additions of bromine to double bonds gave entirely antistereochemistry. Explain why the addition to phenanthrene gives a mixture of synand anti stereochemistry
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 34MP: Using resonance structures of the intermediates, explain why bromination of biphenyl occurs at ortho...
Related questions
Question
most of the additions of bromine to double bonds gave entirely anti
stereochemistry. Explain why the addition to phenanthrene gives a mixture of syn
and anti stereochemistry
Expert Solution
Step 1
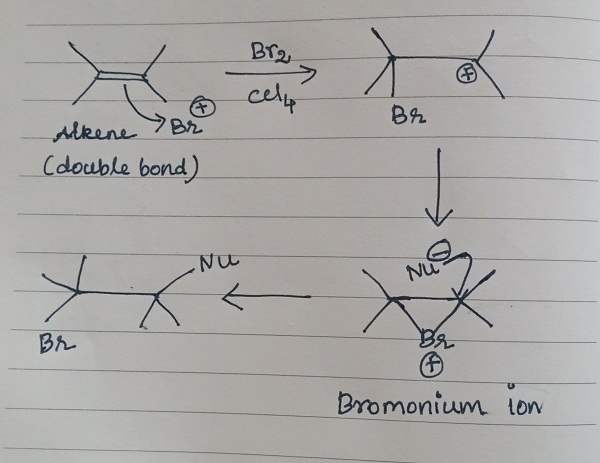
Additions of bromine to double bonds:
In most cases, the additions of bromine to double bonds gave entirely anti stereochemistry, due to the formation of bromonium ion as an intermediate. The nucleophilic attack from the opposite side results in anti-stereochemistry.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning