In the Bohr model of the hydrogen atom, the electron moves in a circular orbit of radius 5.3 × 10-11 m with speed 2.2 × 106 m/s. According to this model, what is the magnetic field at the center of a hydrogen atom due to the motion of the electron? Hint: Determine the average current of the orbiting electron. Express your answer using two significant figures. B ΜΕ ΑΣΦ xa a b xx |X| x.10" 5.127.1011 Submit Previous Answers Request Answer ? ☑ T
In the Bohr model of the hydrogen atom, the electron moves in a circular orbit of radius 5.3 × 10-11 m with speed 2.2 × 106 m/s. According to this model, what is the magnetic field at the center of a hydrogen atom due to the motion of the electron? Hint: Determine the average current of the orbiting electron. Express your answer using two significant figures. B ΜΕ ΑΣΦ xa a b xx |X| x.10" 5.127.1011 Submit Previous Answers Request Answer ? ☑ T
Chapter12: Sources Of Magnetic Fields
Section: Chapter Questions
Problem 25P: A long, straight, horizontal wire carries a left-to-right current of 20 A. If the wire is placed in...
Related questions
Question
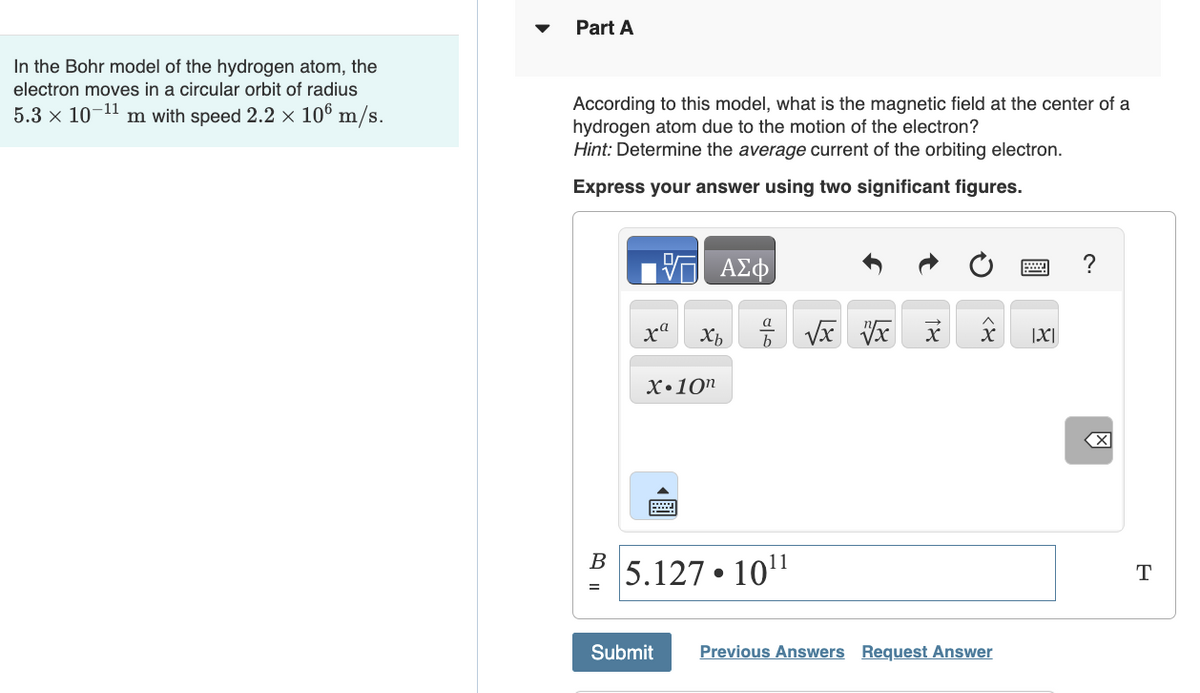
Transcribed Image Text:In the Bohr model of the hydrogen atom, the
electron moves in a circular orbit of radius
5.3 × 10-11 m with speed 2.2 × 106 m/s.
Part A
According to this model, what is the magnetic field at the center of a
hydrogen atom due to the motion of the electron?
Hint: Determine the average current of the orbiting electron.
Express your answer using two significant figures.
B
ΑΣΦ
a
xa
Хь
b
% √x x x
X.10n
5.127.1011
<४
Submit
Previous Answers Request Answer
|X|
?
☑
T
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning