I do X Tabroom.c X Tuesday 3 X Iné Fanati And Fanati X vault 4n6fix || W Daughter P * || W chemquiz.net/sto/ Prodigy Caddo Mills (SD/Q Google Docs ■Google Slides Gmail Jostens Yearbook A. 1. Problem 2 NH3 + 3 12 → N2 + 6 HI If 1.6509 moles of HI are produced, how many grams of 12 were reacted? 2. F2+2 KCI 2 KF + Cl2 If 3.4983 moles of KCI are reacted, how many moles of Cl₂ will be produce 3. H2SO4 + Fe FeSO4 + H2 4. If 1.93 moles of H2 are produced, how many moles of H2SO4 were reacted C2H4 + H2 C2H6 If 2.0462 moles of C2H6 are produced, how many moles of C2H4 were rea 5. H₂SO4 + Ba(OH)2 → BaSO4 + 2 H₂O 6. If 313 grams of BaSO4 are produced, how many grams of H2SO4 were re- SiC + 2 Cl₂ SiCl4 + C If 350 ms of
I do X Tabroom.c X Tuesday 3 X Iné Fanati And Fanati X vault 4n6fix || W Daughter P * || W chemquiz.net/sto/ Prodigy Caddo Mills (SD/Q Google Docs ■Google Slides Gmail Jostens Yearbook A. 1. Problem 2 NH3 + 3 12 → N2 + 6 HI If 1.6509 moles of HI are produced, how many grams of 12 were reacted? 2. F2+2 KCI 2 KF + Cl2 If 3.4983 moles of KCI are reacted, how many moles of Cl₂ will be produce 3. H2SO4 + Fe FeSO4 + H2 4. If 1.93 moles of H2 are produced, how many moles of H2SO4 were reacted C2H4 + H2 C2H6 If 2.0462 moles of C2H6 are produced, how many moles of C2H4 were rea 5. H₂SO4 + Ba(OH)2 → BaSO4 + 2 H₂O 6. If 313 grams of BaSO4 are produced, how many grams of H2SO4 were re- SiC + 2 Cl₂ SiCl4 + C If 350 ms of
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 120AP
Related questions
Question
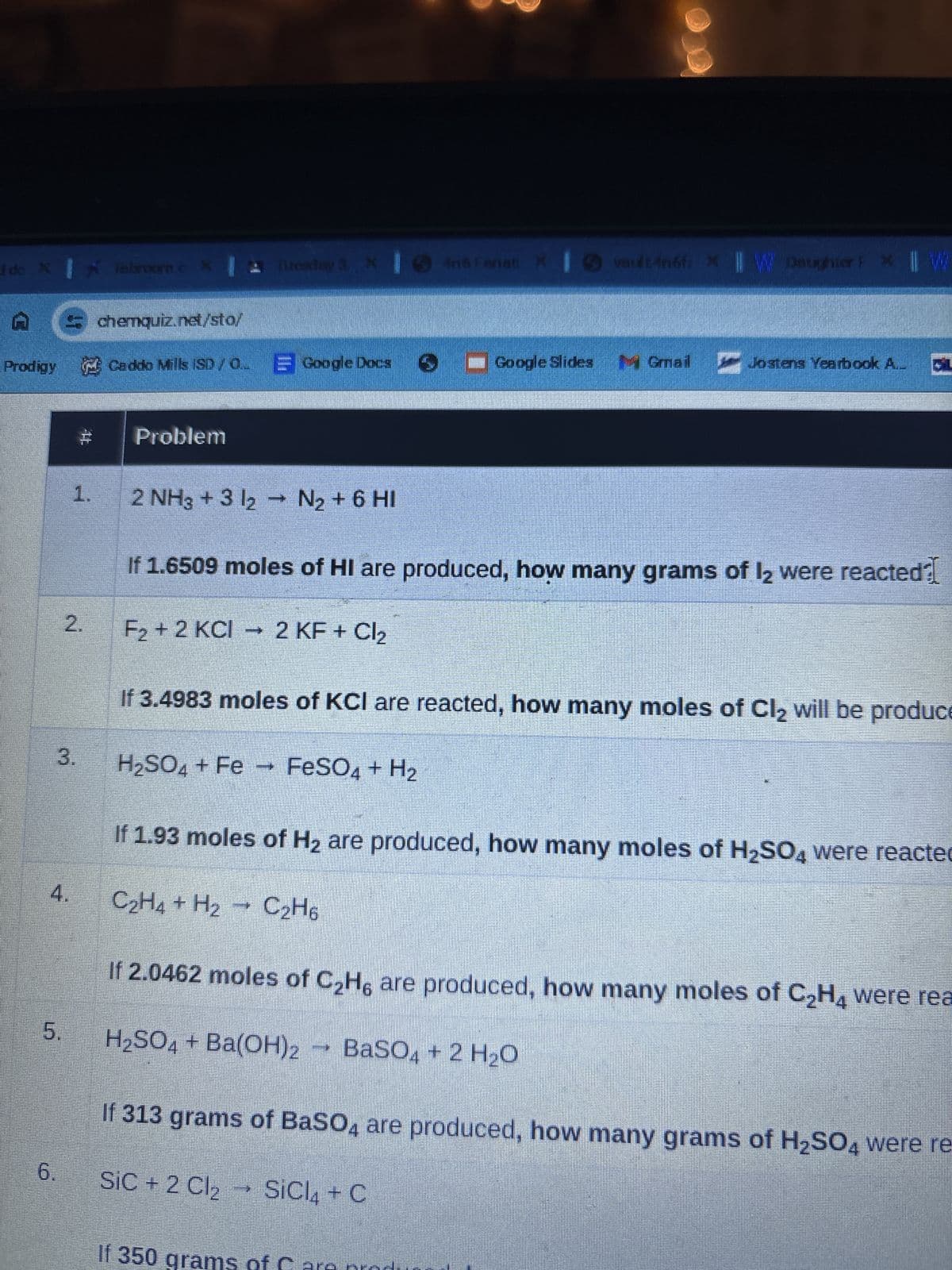
Transcribed Image Text:I do X Tabroom.c X Tuesday 3 X Iné Fanati
And Fanati X
vault 4n6fix || W Daughter P * || W
chemquiz.net/sto/
Prodigy Caddo Mills (SD/Q Google Docs ■Google Slides Gmail Jostens Yearbook A.
1.
Problem
2 NH3 + 3 12 → N2 + 6 HI
If 1.6509 moles of HI are produced, how many grams of 12 were reacted?
2.
F2+2 KCI 2 KF + Cl2
If 3.4983 moles of KCI are reacted, how many moles of Cl₂ will be produce
3.
H2SO4 + Fe FeSO4 + H2
4.
If 1.93 moles of H2 are produced, how many moles of H2SO4 were reacted
C2H4 + H2 C2H6
If 2.0462 moles of C2H6 are produced, how many moles of C2H4 were rea
5.
H₂SO4 + Ba(OH)2 → BaSO4 + 2 H₂O
6.
If 313 grams of BaSO4 are produced, how many grams of H2SO4 were re-
SiC + 2 Cl₂
SiCl4 + C
If 350
ms of
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning