For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find Ks, data in the ALEKS Data tab. sp compound Does solubility change with highest solubility pH? pH = 5 pH = 6 pH = 8 yes no yes 12 12 yes CaBr, no O O ZnS ? 00. 18 Ar
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the solubility does change, pick the pH at which you'd expect the highest solubility. You'll find Ks, data in the ALEKS Data tab. sp compound Does solubility change with highest solubility pH? pH = 5 pH = 6 pH = 8 yes no yes 12 12 yes CaBr, no O O ZnS ? 00. 18 Ar
Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.22QAP
Related questions
Question
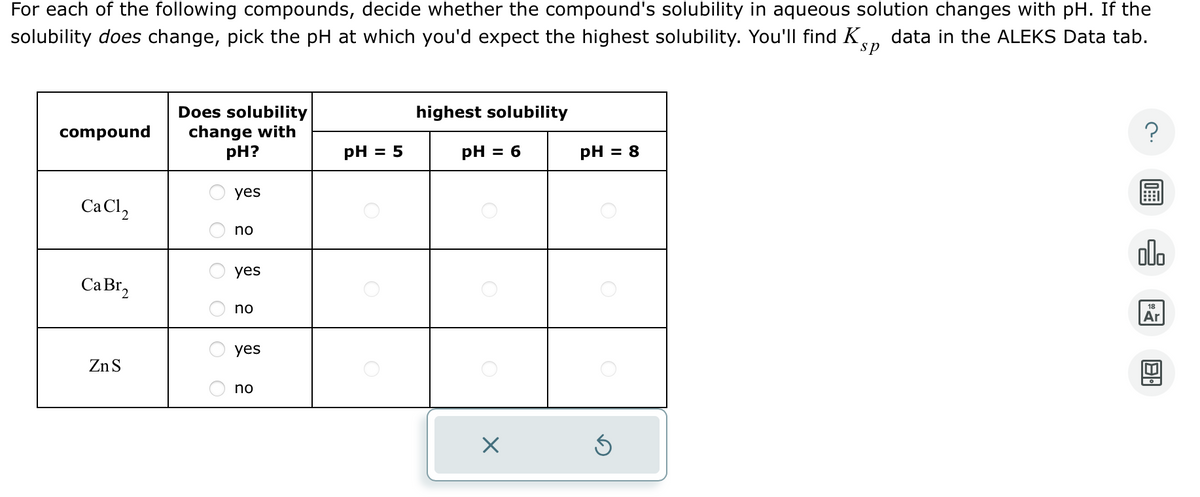
Transcribed Image Text:For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with pH. If the
solubility does change, pick the pH at which you'd expect the highest solubility. You'll find Ks, data in the ALEKS Data tab.
sp
compound
Does solubility
change with
highest solubility
pH?
pH = 5
pH = 6
pH = 8
yes
no
yes
12 12
yes
CaBr,
no
O O
ZnS
?
00.
18
Ar
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax