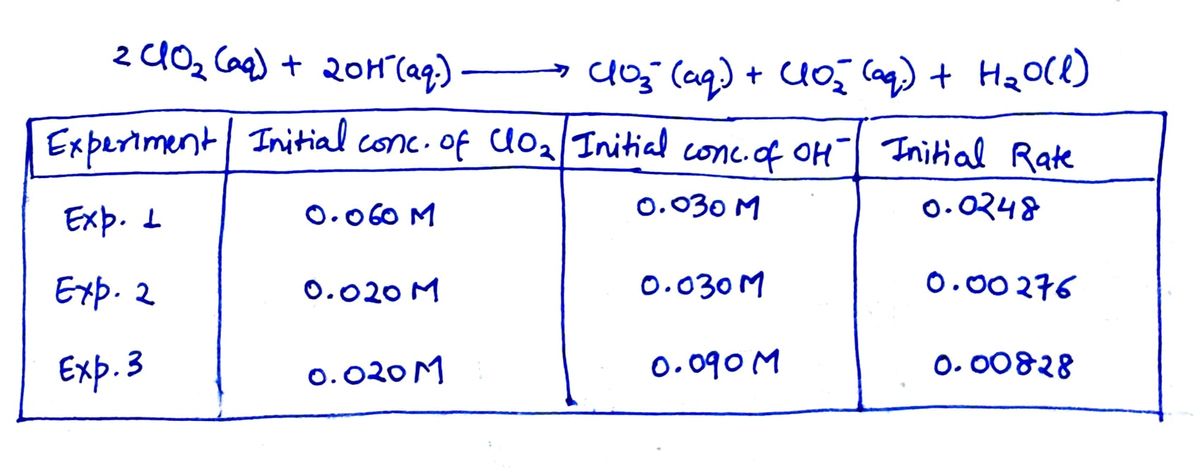
Chlorine dioxide, ClO2, is a reddish-yellow gas that is soluble in water, In basic solution it gives ClO3- and ClO2- ions. 2ClO2(aq) + 2OH-(aq) → ClO3-(aq) + ClO2-(aq) + H2O(l) To obtain the rate law for this reaction, the following experiments were run and, for each, the intitial rate of reaction of ClO2 was determined. Expriment Initial Conc. of ClO2 Initial Conc. of OH- Initial Rate (mol/(L•s)) Exp. 1 0.060 M 0.030 M 0.0248 Exp. 2 0.020 M 0.030 M 0.00276 Exp. 3 0.020 M 0.090 M 0.00828 What is the rate law for the reaction? Input the answer in the form rate = k[A]2[B]
Chlorine dioxide, ClO2, is a reddish-yellow gas that is soluble in water, In basic solution it gives ClO3- and ClO2- ions. 2ClO2(aq) + 2OH-(aq) → ClO3-(aq) + ClO2-(aq) + H2O(l) To obtain the rate law for this reaction, the following experiments were run and, for each, the intitial rate of reaction of ClO2 was determined. Expriment Initial Conc. of ClO2 Initial Conc. of OH- Initial Rate (mol/(L•s)) Exp. 1 0.060 M 0.030 M 0.0248 Exp. 2 0.020 M 0.030 M 0.00276 Exp. 3 0.020 M 0.090 M 0.00828 What is the rate law for the reaction? Input the answer in the form rate = k[A]2[B]
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter13: Rates Of Reaction
Section: Chapter Questions
Problem 13.55QP: Chlorine dioxide, ClO2, is a reddish-yellow gas that is soluble in water. In basic solution it gives...
Related questions
Question
Chlorine dioxide, ClO2, is a reddish-yellow gas that is soluble in water, In basic solution it gives ClO3- and ClO2- ions.
2ClO2(aq) + 2OH-(aq) → ClO3-(aq) + ClO2-(aq) + H2O(l)
To obtain the rate law for this reaction, the following experiments were run and, for each, the intitial
| Expriment |
Initial Conc. of ClO2 |
Initial Conc. of OH- |
Initial Rate (mol/(L•s)) |
| Exp. 1 | 0.060 M | 0.030 M | 0.0248 |
| Exp. 2 | 0.020 M | 0.030 M | 0.00276 |
| Exp. 3 | 0.020 M | 0.090 M | 0.00828 |
What is the rate law for the reaction?
Input the answer in the form rate = k[A]2[B]
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning