An Introduction to Physical Science
14th Edition
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Chapter4: Work And Energy
Section: Chapter Questions
Problem 14FIB: Gasohol is gasoline mixed with ___ . (4.6)
Related questions
Question
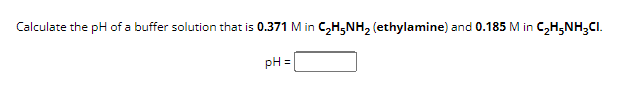
Transcribed Image Text:Calculate the pH of a buffer solution that is 0.371 M in C₂H5NH2 (ethylamine) and 0.185 M in C₂H5NH3CI.
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps with 1 images

Recommended textbooks for you

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning


An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning
