Below, write the balanced chemical equation for the reaction you observed (including state). If a precipitate formed, you will be shown the formula of that new compound, plus one of the left-over ions (on the right side of the screen). The nitrate ion was not displayed on the projection, but assume it is also present. Write the balanced chemical equation for this reaction. Each reactant and product should be written as a neutral compound. Use
Below, write the balanced chemical equation for the reaction you observed (including state). If a precipitate formed, you will be shown the formula of that new compound, plus one of the left-over ions (on the right side of the screen). The nitrate ion was not displayed on the projection, but assume it is also present. Write the balanced chemical equation for this reaction. Each reactant and product should be written as a neutral compound. Use
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter5: Chemical Reactions
Section: Chapter Questions
Problem 5.62E: Rewrite the following word equation using chemical formulas. Balance the equation and write the...
Related questions
Question
Below, write the balanced chemical equation for the reaction you observed (including state).
If a precipitate formed, you will be shown the formula of that new compound, plus one of
the left-over ions (on the right side of the screen). The nitrate ion was not displayed on the
projection, but assume it is also present. Write the balanced chemical equation for this reaction.
Each reactant and product should be written as a neutral compound. Use your observations and the
solubility rules to determine the state for each compound, (s) or (aq).
PROPOSED BALANCED CHEMICAL EQUATION
Pb(NO3)2 + NaCl
Expert Solution
Step 1
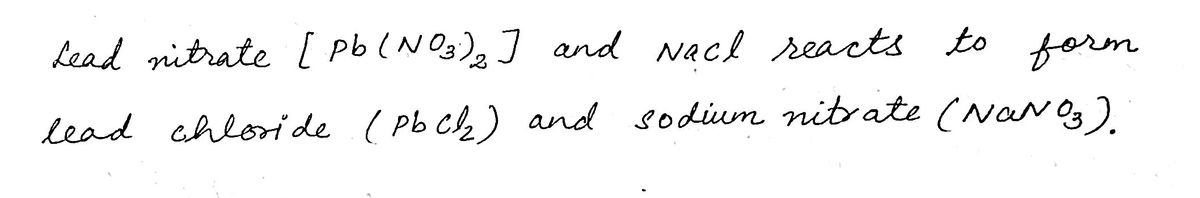
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning