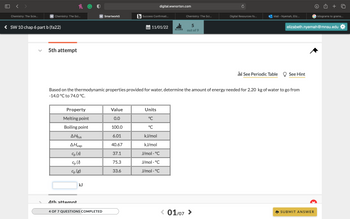
Based on the thermodynamic properties provided for water, determine the amount of energy needed for 2.20 kg of water to go from -14.0 °C to 74.0 °C.
Based on the thermodynamic properties provided for water, determine the amount of energy needed for 2.20 kg of water to go from -14.0 °C to 74.0 °C.
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter20: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 20.12OQ: If a gas is compressed isothermally, which of the following statements is true? (a) Energy is...
Related questions
Question
Based on the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Answer was incorrect, but i tried plugging in other values for Cp and they didn't work, is there another step to the problem
Transcribed Image Text:Chemistry: The Scie...
W Chemistry: The Sci...
< SW 10 chap 6 part b (fa22)
5th attempt
h G
Property
Melting point
Boiling point
AHfus
AHvap
Cp (s)
Cp (1)
Cp (8)
4th attemnt
W Smartwork5
kJ
4 OF 7 QUESTIONS COMPLETED
digital.wwnorton.com
Value
0.0
100.0
6.01
40.67
37.1
75.3
33.6
b Success Confirmati...
11/01/22 J
Chemistry: The Sci...
5
SCORE out of 9
Based on the thermodynamic properties provided for water, determine the amount of energy needed for 2.20 kg of water to go from
-14.0 °C to 74.0 °C.
Units
°℃
°C
kJ/mol
kJ/mol
J/mol • °C
J/mol • °C
J/mol • °C
Digital Resources fo...
< 01/07 >
Mail - Nyemah, Eliz...
.::See Periodic Table
elizabeth.nyemah@mnsu.edu
See Hint
kilograms to grams...
SUBMIT ANSWER
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning