Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter11: Stoichiometry
Section: Chapter Questions
Problem 43A
Related questions
Question
Please help fill in the rest of the table using the provided data. Show your work.

Transcribed Image Text:|MKMnO₂ = -0.0101 M
Weight of NaBO3 4H₂O(g)
Vol. NaBO3 4H₂O used (mL)
Moles NaBO3 4H₂O (mol)*
Initial buret reading (mL)
Final buret reading (mL)
Volume KMnO4 used (mL)
Moles KMnO4 used (mol)*
Calculated mole ratio (from
starred *values, above) -
i.e. H₂O₂: MnO4
Trial 1
1.0062
10.00
0.0
24.04
24.04
Trial 2
(same) 1
10.00
24.07
24.07
Trial 3
(same) 1
10.00
0.0
24.07
24.07
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Based on the previously asked question and it's data, please help fill in the rest of the table. Show your work. The molarity of KMnO4 is 0.0101M.
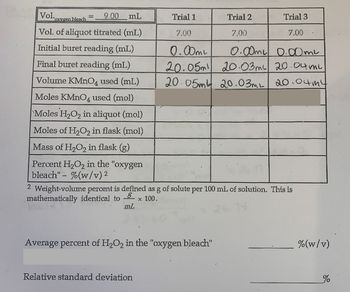
Transcribed Image Text:Vol.xver
9.00 mL
"Oxygen bleach
Vol. of aliquot titrated (mL)
Initial buret reading (mL)
Final buret reading (mL)
Volume KMnO4 used (mL)
Moles KMnO4 used (mol)
Moles H₂O₂ in aliquot (mol)
Moles of H₂O₂ in flask (mol)
Mass of H₂O₂ in flask (g)
Percent H₂O₂ in the "oxygen
bleach" - %(w/v) ²
2
mL
Trial 1
Trial 3
7.00
7.00
0.00ml
0.00ml 0.00mL
20.05m 20.03m² 20.04 mi
20.05m 20.03mL 20.04 my
2 Weight-volume percent is defined as g of solute per 100 mL of solution. This is
mathematically identical to
× 100.
Average percent of H₂O2 in the "oxygen bleach"
Relative standard deviation
Trial 2
7.00
%(w/v)
%
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

