A Moving to another question will save this response. Question 7 Which of the following statements NOT an assumption of the ideal gas law? a. The collision between molecules is elastic. Ob. The intermolecular forces between individual molecules keep them distant. c. Gas molecules are infinitely small and have no significant volume. d. Gas molecules are always in motion A Moving to another question will save this response.
A Moving to another question will save this response. Question 7 Which of the following statements NOT an assumption of the ideal gas law? a. The collision between molecules is elastic. Ob. The intermolecular forces between individual molecules keep them distant. c. Gas molecules are infinitely small and have no significant volume. d. Gas molecules are always in motion A Moving to another question will save this response.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 22ALQ
Related questions
Question
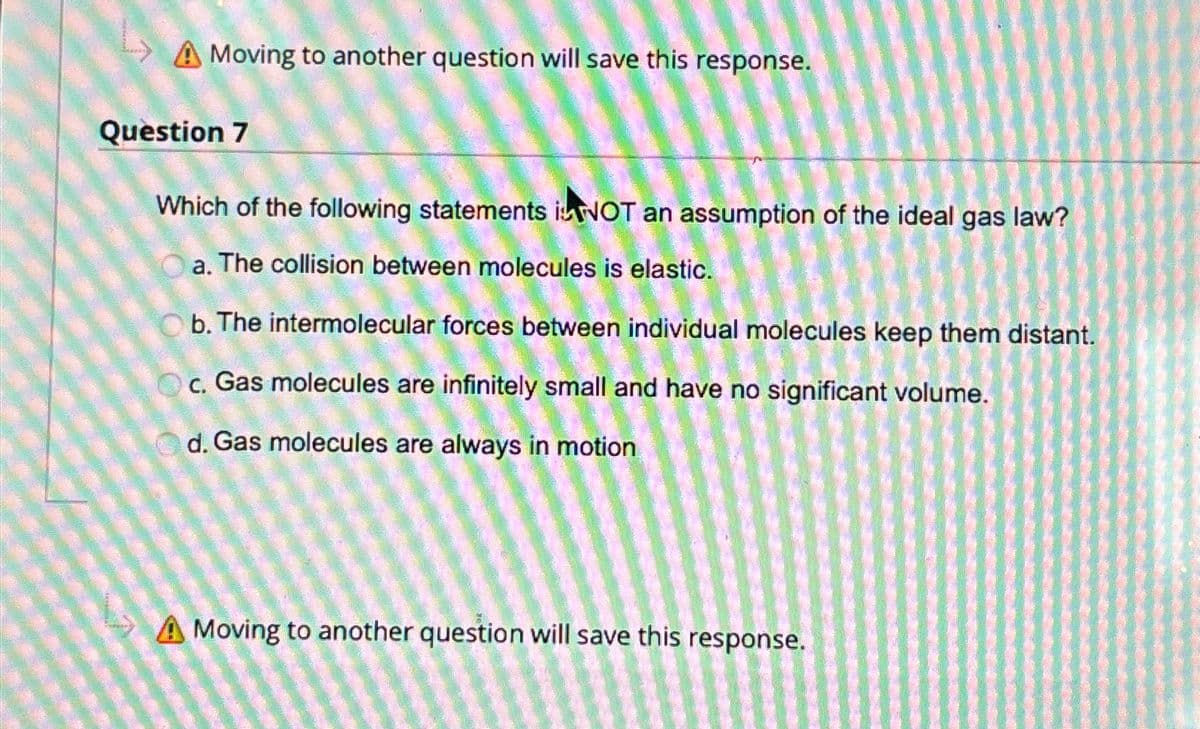
Transcribed Image Text:A Moving to another question will save this response.
Question 7
Which of the following statements NOT an assumption of the ideal gas law?
a. The collision between molecules is elastic.
Ob. The intermolecular forces between individual molecules keep them distant.
c. Gas molecules are infinitely small and have no significant volume.
d. Gas molecules are always in motion
A Moving to another question will save this response.
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning