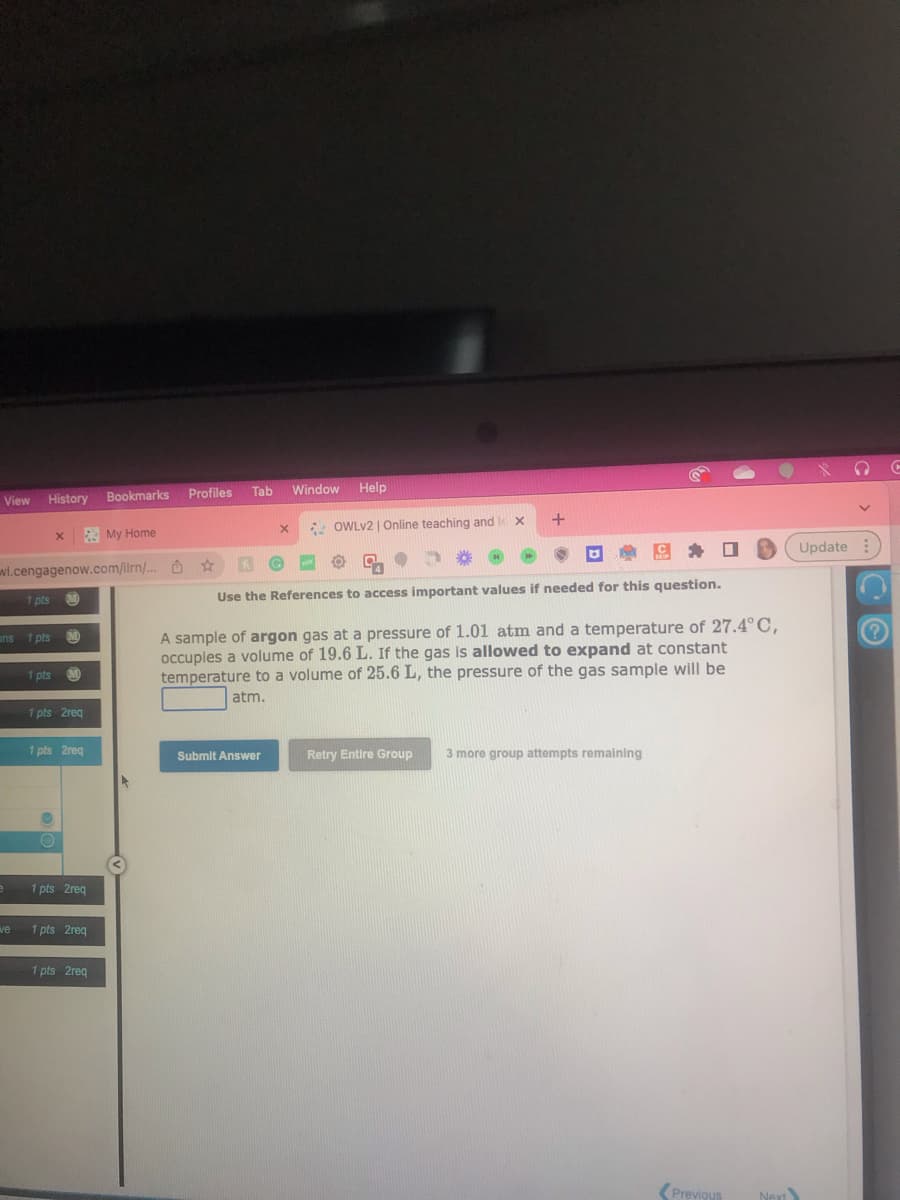
_.com/ilrn/... Use the References to access important values if needed for this question. A sample of argon gas at a pressure of 1.01 atm and a temperature of 27.4°C, occupies a volume of 19.6 L. If the gas is allowed to expand at constant temperature to a volume of 25.6 L, the pressure of the gas sample will be atm. Submit Answer Retry Entire Group 3 more group attempts remaining Update
_.com/ilrn/... Use the References to access important values if needed for this question. A sample of argon gas at a pressure of 1.01 atm and a temperature of 27.4°C, occupies a volume of 19.6 L. If the gas is allowed to expand at constant temperature to a volume of 25.6 L, the pressure of the gas sample will be atm. Submit Answer Retry Entire Group 3 more group attempts remaining Update
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter7: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 7.22EP
Related questions
Question
100%
Help
Transcribed Image Text:View
History
ons 1 pts
ve
X
wwl.cengagenow.com/ilrn/...
1 pts M
1 pts
M
1 pts 2req
1 pts 2req
O
1 pts 2req
1 pts 2req
Bookmarks
1 pts 2req
My Home
Profiles
Tab Window Help
X
OWLv2 | Online teaching and
Submit Answer
CI
Use the References to access important values if needed for this question.
O
X +
A sample of argon gas at a pressure of 1.01 atm and a temperature of 27.4°C,
occupies a volume of 19.6 L. If the gas is allowed to expand at constant
temperature to a volume of 25.6 L, the pressure of the gas sample will be
atm.
Retry Entire Group
3 more group attempts remaining
<Previous
Next
Update:
?
C
Transcribed Image Text:story
M
now.com/ilrn/...
M
M
2req
2req
2req
2req
Bookmarks
2req
My Home
Visited
Profiles
Tab
X
Window
Submit Answer
Help
OWLv2 | Online teaching and le X
+
★口
Use the References to access important values if needed for this question.
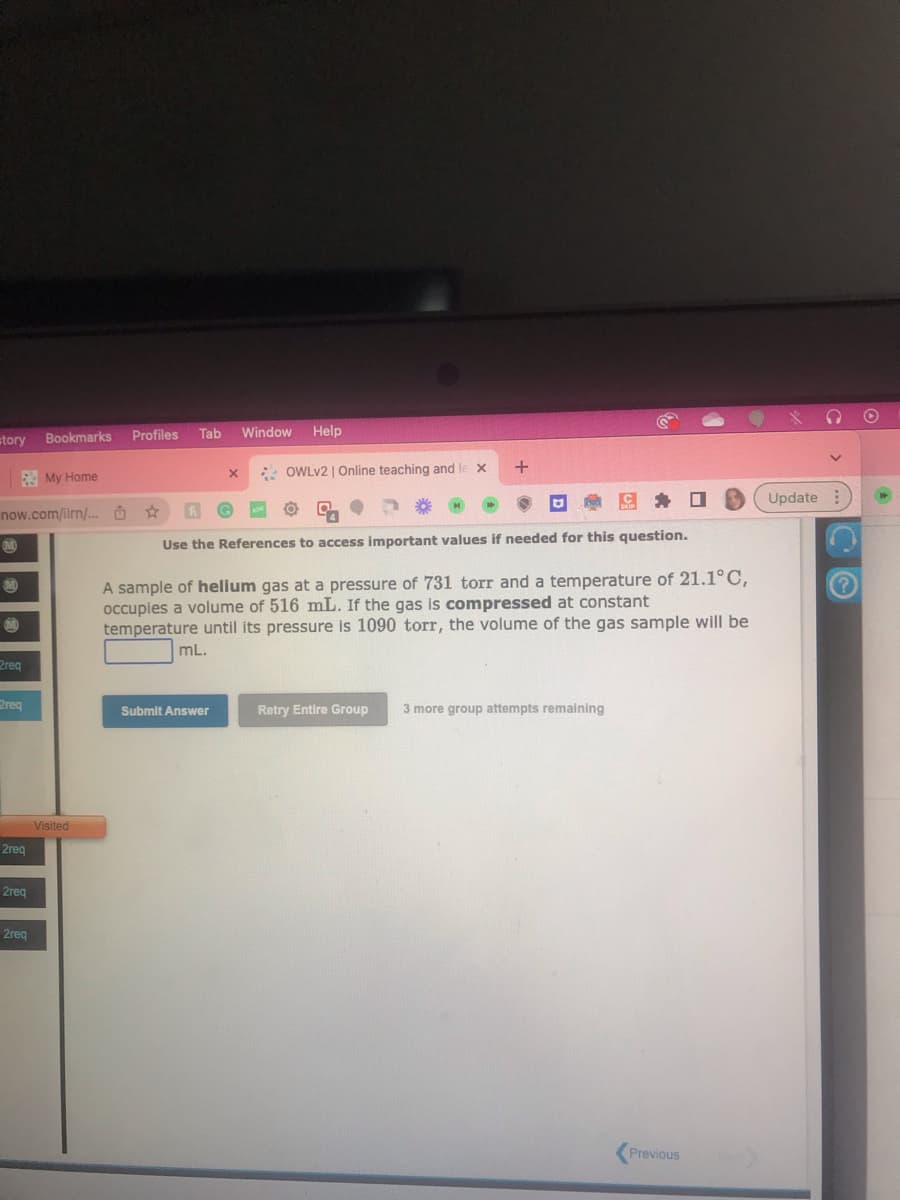
A sample of helium gas at a pressure of 731 torr and a temperature of 21.1°C,
occupies a volume of 516 mL. If the gas is compressed at constant
temperature until its pressure is 1090 torr, the volume of the gas sample will be
mL.
Retry Entire Group 3 more group attempts remaining
Previous
Update
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning