6:30 PM Mon Mar 25 ED Calculate the pH at the equivalence point for the following titrations. 4A. Benzoic 50. mL of 0.12 M benzoic acid titrated by 0.10 M potassium hydroxide = ka C6H5COOH 6.3 x 10-5 4B. 50. mL of 0.15 M ethylamine titrated by 0.20 M chloric acid Ethylamine CH3CH2NH2 6.4 x 104 K
6:30 PM Mon Mar 25 ED Calculate the pH at the equivalence point for the following titrations. 4A. Benzoic 50. mL of 0.12 M benzoic acid titrated by 0.10 M potassium hydroxide = ka C6H5COOH 6.3 x 10-5 4B. 50. mL of 0.15 M ethylamine titrated by 0.20 M chloric acid Ethylamine CH3CH2NH2 6.4 x 104 K
Chapter14: Principles Of Neutralization Titrations
Section: Chapter Questions
Problem 14.6QAP
Related questions
Question
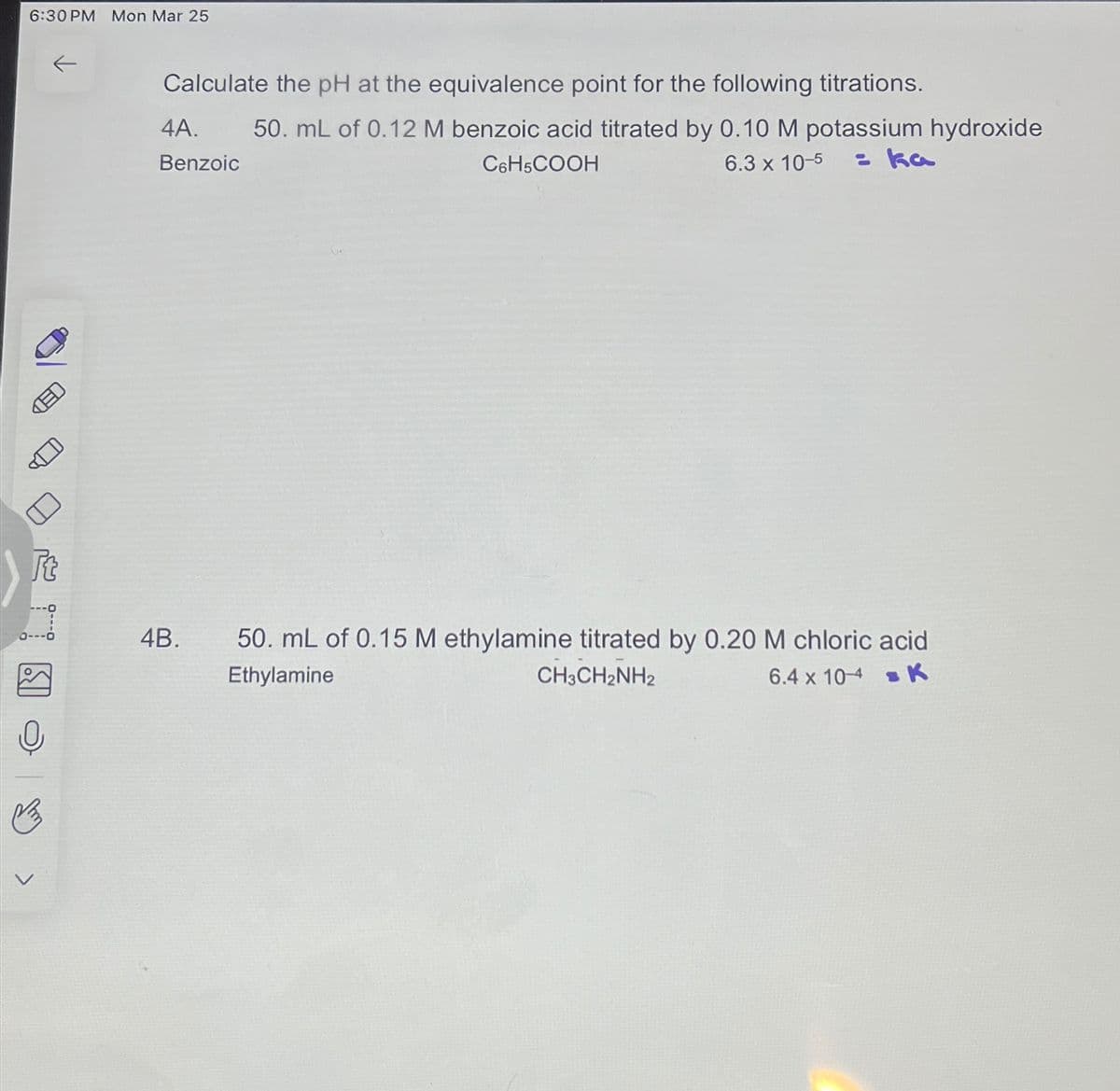
Transcribed Image Text:6:30 PM Mon Mar 25
ED
Calculate the pH at the equivalence point for the following titrations.
4A.
Benzoic
50. mL of 0.12 M benzoic acid titrated by 0.10 M potassium hydroxide
= ka
C6H5COOH
6.3 x 10-5
4B.
50. mL of 0.15 M ethylamine titrated by 0.20 M chloric acid
Ethylamine
CH3CH2NH2
6.4 x 104 K
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning